Gene Body Methylation in Plants: Mechanisms, Functions, and Important Implications for Understanding Evolutionary Processes
- PMID: 35298639
- PMCID: PMC8995044
- DOI: 10.1093/gbe/evac038
Gene Body Methylation in Plants: Mechanisms, Functions, and Important Implications for Understanding Evolutionary Processes
Abstract
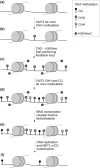
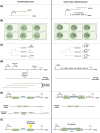
Gene body methylation (gbM) is an epigenetic mark where gene exons are methylated in the CG context only, as opposed to CHG and CHH contexts (where H stands for A, C, or T). CG methylation is transmitted transgenerationally in plants, opening the possibility that gbM may be shaped by adaptation. This presupposes, however, that gbM has a function that affects phenotype, which has been a topic of debate in the literature. Here, we review our current knowledge of gbM in plants. We start by presenting the well-elucidated mechanisms of plant gbM establishment and maintenance. We then review more controversial topics: the evolution of gbM and the potential selective pressures that act on it. Finally, we discuss the potential functions of gbM that may affect organismal phenotypes: gene expression stabilization and upregulation, inhibition of aberrant transcription (reverse and internal), prevention of aberrant intron retention, and protection against TE insertions. To bolster the review of these topics, we include novel analyses to assess the effect of gbM on transcripts. Overall, a growing body of literature finds that gbM correlates with levels and patterns of gene expression. It is not clear, however, if this is a causal relationship. Altogether, functional work suggests that the effects of gbM, if any, must be relatively small, but there is nonetheless evidence that it is shaped by natural selection. We conclude by discussing the potential adaptive character of gbM and its implications for an updated view of the mechanisms of adaptation in plants.
Keywords: DNA methylation; epigenetics; gene expression; population epigenomics; transcription.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures


References
-
- Arias T, Beilstein MA, Tang M, McKain MR, Pires JC. 2014. Diversification times among Brassica (Brassicaceae) crops suggest hybrid formation after 20 million years of divergence. Am J Bot. 101:86–91. - PubMed

