Protein tyrosine phosphatase receptor δ serves as the orexigenic asprosin receptor
- PMID: 35298903
- PMCID: PMC8986618
- DOI: 10.1016/j.cmet.2022.02.012
Protein tyrosine phosphatase receptor δ serves as the orexigenic asprosin receptor
Abstract
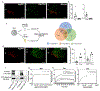
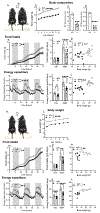
Asprosin is a fasting-induced glucogenic and centrally acting orexigenic hormone. The olfactory receptor Olfr734 is known to be the hepatic receptor for asprosin that mediates its effects on glucose production, but the receptor for asprosin's orexigenic function has been unclear. Here, we have identified protein tyrosine phosphatase receptor δ (Ptprd) as the orexigenic receptor for asprosin. Asprosin functions as a high-affinity Ptprd ligand in hypothalamic AgRP neurons, regulating the activity of this circuit in a cell-autonomous manner. Genetic ablation of Ptprd results in a strong loss of appetite, leanness, and an inability to respond to the orexigenic effects of asprosin. Ablation of Ptprd specifically in AgRP neurons causes resistance to diet-induced obesity. Introduction of the soluble Ptprd ligand-binding domain in the circulation of mice suppresses appetite and blood glucose levels by sequestering plasma asprosin. Identification of Ptprd as the orexigenic asprosin receptor creates a new avenue for the development of anti-obesity therapeutics.
Keywords: AgRP; Ptprd; appetite; asprosin; hypothalamus; metabolism; obesity; receptor.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Atul Chopra has been awarded asprosin-related patents and is a co-founder, director, and officer of Vizigen, Inc. and Aceragen, Inc. and holds equity in both companies. The other authors declare no competing interests.
Figures

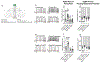



References
-
- Alan M, Gurlek B, Yilmaz A, Aksit M, Aslanipour B, Gulhan I, Mehmet C, and Taner CE (2018). Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol 35, 1–4. - PubMed
-
- Baykus Y, Yavuzkir S, Ustebay S, Ugur K, Deniz R, and Aydin S (2019). Asprosin in umbilical cord of newborns and maternal blood of gestational diabetes, preeclampsia, severe preeclampsia, intrauterine growth retardation and macrosemic fetus. Peptides 120, 170132. - PubMed
-
- Bienvenu T, Lebrun N, Clarke J, Duriez P, Gorwood P, and Ramoz N (2020). De novo deleterious variants that may alter the dopaminergic reward pathway are associated with anorexia nervosa. Eat Weight Disord - Stud Anorexia Bulimia Obes 25, 1643–1650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

