High-throughput biochemical profiling reveals functional adaptation of a bacterial Argonaute
- PMID: 35298909
- PMCID: PMC9158488
- DOI: 10.1016/j.molcel.2022.02.026
High-throughput biochemical profiling reveals functional adaptation of a bacterial Argonaute
Abstract
Argonautes are nucleic acid-guided proteins that perform numerous cellular functions across all domains of life. Little is known about how distinct evolutionary pressures have shaped each Argonaute's biophysical properties. We applied high-throughput biochemistry to characterize how Thermus thermophilus Argonaute (TtAgo), a DNA-guided DNA endonuclease, finds, binds, and cleaves its targets. We found that TtAgo uses biophysical adaptations similar to those of eukaryotic Argonautes for rapid association but requires more extensive complementarity to achieve high-affinity target binding. Using these data, we constructed models for TtAgo association rates and equilibrium binding affinities that estimate the nucleic acid- and protein-mediated components of the target interaction energies. Finally, we showed that TtAgo cleavage rates vary widely based on the DNA guide, suggesting that only a subset of guides cleaves targets on physiologically relevant timescales.
Keywords: Argonaute; RNA silencing; Thermus thermophilus; TtAgo; high-throughput biochemistry; miRNA; pAGO; siDNA; siRNA; single-molecule biophysics.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.D.Z. is a member of the scientific advisory boards of Alnylam Pharmaceuticals, Voyager Therapeutics, and ProQR. He is also a consultant for The RNA Medicines Company. W.J.G is a scientific co-founder of Protillion and a consultant, and equity holder, for Guardant Health, 10X Genomics, Ultima Genomics, and Quantapore.
Figures
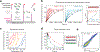


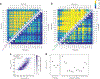


Similar articles
-
Thermus thermophilus Argonaute Functions in the Completion of DNA Replication.Cell. 2020 Sep 17;182(6):1545-1559.e18. doi: 10.1016/j.cell.2020.07.036. Epub 2020 Aug 25. Cell. 2020. PMID: 32846159 Free PMC article.
-
Autonomous Generation and Loading of DNA Guides by Bacterial Argonaute.Mol Cell. 2017 Mar 16;65(6):985-998.e6. doi: 10.1016/j.molcel.2017.01.033. Epub 2017 Mar 2. Mol Cell. 2017. PMID: 28262506 Free PMC article.
-
Effects of Argonaute on Gene Expression in Thermus thermophilus.PLoS One. 2015 Apr 22;10(4):e0124880. doi: 10.1371/journal.pone.0124880. eCollection 2015. PLoS One. 2015. PMID: 25902012 Free PMC article.
-
Understanding the core of RNA interference: The dynamic aspects of Argonaute-mediated processes.Prog Biophys Mol Biol. 2017 Sep;128:39-46. doi: 10.1016/j.pbiomolbio.2016.09.008. Epub 2016 Sep 30. Prog Biophys Mol Biol. 2017. PMID: 27697475 Review.
-
Argonaute proteins: structures and their endonuclease activity.Mol Biol Rep. 2021 May;48(5):4837-4849. doi: 10.1007/s11033-021-06476-w. Epub 2021 Jun 11. Mol Biol Rep. 2021. PMID: 34117606 Review.
Cited by
-
A tiny loop in the Argonaute PIWI domain tunes small RNA seed strength.EMBO Rep. 2023 Jun 5;24(6):e55806. doi: 10.15252/embr.202255806. Epub 2023 Apr 21. EMBO Rep. 2023. PMID: 37082939 Free PMC article.
-
The structural basis for RNA slicing by human Argonaute2.bioRxiv [Preprint]. 2024 Aug 20:2024.08.19.608718. doi: 10.1101/2024.08.19.608718. bioRxiv. 2024. Update in: Cell Rep. 2025 Jan 28;44(1):115166. doi: 10.1016/j.celrep.2024.115166. PMID: 39229170 Free PMC article. Updated. Preprint.
-
The structural basis for RNA slicing by human Argonaute2.Cell Rep. 2025 Jan 28;44(1):115166. doi: 10.1016/j.celrep.2024.115166. Epub 2024 Dec 31. Cell Rep. 2025. PMID: 39932188 Free PMC article.
-
Systematic analysis of cotranscriptional RNA folding using transcription elongation complex display.Nat Commun. 2025 Mar 10;16(1):2350. doi: 10.1038/s41467-025-57415-3. Nat Commun. 2025. PMID: 40064876 Free PMC article.
-
Characterization of argonaute nucleases from mesophilic bacteria Pseudobutyrivibrio ruminis.Bioresour Bioprocess. 2024 Oct 7;11(1):94. doi: 10.1186/s40643-024-00797-x. Bioresour Bioprocess. 2024. PMID: 39373873 Free PMC article.
References
-
- Ameres SL, Martinez J, and Schroeder R. (2007). Molecular basis for target RNA recognition and cleavage by human RISC. Cell 130, 101–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

