Cystic Neoplasms of the Pancreas: Differential Diagnosis and Radiology Correlation
- PMID: 35299739
- PMCID: PMC8921498
- DOI: 10.3389/fonc.2022.860740
Cystic Neoplasms of the Pancreas: Differential Diagnosis and Radiology Correlation
Abstract
Although the probability of pancreatic cystic neoplasms (PCNs) being detected is raising year by year, their differential diagnosis and individualized treatment are still a challenge in clinical work. PCNs are tumors containing cystic components with different biological behaviors, and their clinical manifestations, epidemiology, imaging features, and malignant risks are different. Some are benign [e.g., serous cystic neoplasms (SCNs)], with a barely possible that turning into malignant, while others display a low or higher malignant risk [e.g., solid pseudopapillary neoplasms (SPNs), intraductal papillary mucinous neoplasms (IPMNs), and mucinous cystic neoplasms (MCNs)]. PCN management should concentrate on preventing the progression of malignant tumors while preventing complications caused by unnecessary surgical intervention. Clinically, various advanced imaging equipment are usually combined to obtain a more reliable preoperative diagnosis. The challenge for clinicians and radiologists is how to accurately diagnose PCNs before surgery so that corresponding surgical methods and follow-up strategies can be developed or not, as appropriate. The objective of this review is to sum up the clinical features, imaging findings and management of the most common PCNs according to the classic literature and latest guidelines.
Keywords: intraductal papillary mucinous neoplasms (IPMNs); mucinous cystic neoplasms (MCNs); pancreatic cystic neoplasms (PCNs); radiology; serous cystic neoplasms (SCN); solid pseudopapillary neoplasms (SPN).
Copyright © 2022 Hu, Hu, Wang, Ma, Yue, Tang, Liu, Wu, Peng and Tong.
Conflict of interest statement
Author PW was employed by GE Healthcare. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

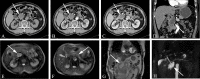
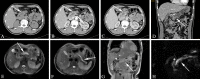
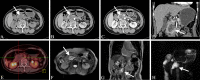
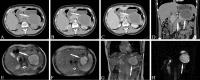
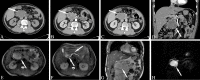
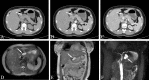
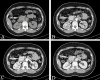
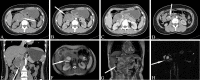
Similar articles
-
Pancreatic cystic neoplasms: a review of preoperative diagnosis and management.J Zhejiang Univ Sci B. 2013 Mar;14(3):185-94. doi: 10.1631/jzus.B1200283. J Zhejiang Univ Sci B. 2013. PMID: 23463761 Free PMC article. Review.
-
Cystic neoplasms of the exocrine pancreas: an update of a nationwide survey in Korea.Pancreas. 2008 Oct;37(3):254-8. doi: 10.1097/MPA.0b013e3181676ba4. Pancreas. 2008. PMID: 18815545
-
Management Algorithms for Pancreatic Cystic Neoplasms.Arch Pathol Lab Med. 2022 Mar 1;146(3):322-329. doi: 10.5858/arpa.2020-0395-RA. Arch Pathol Lab Med. 2022. PMID: 33503225 Review.
-
Patients with a resected pancreatic mucinous cystic neoplasm have a better prognosis than patients with an intraductal papillary mucinous neoplasm: A large single institution series.Pancreatology. 2017 May-Jun;17(3):490-496. doi: 10.1016/j.pan.2017.04.003. Epub 2017 Apr 11. Pancreatology. 2017. PMID: 28416122
-
Radiomics advances in the evaluation of pancreatic cystic neoplasms.Heliyon. 2024 Jan 30;10(3):e25535. doi: 10.1016/j.heliyon.2024.e25535. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38333791 Free PMC article. Review.
Cited by
-
A case of serous cystic neoplasm with tumor growth acceleration leading to extrapancreatic invasion.Clin J Gastroenterol. 2023 Apr;16(2):289-296. doi: 10.1007/s12328-022-01746-x. Epub 2022 Dec 12. Clin J Gastroenterol. 2023. PMID: 36507956
-
A Unique Presentation of Solid Pseudopapillary Neoplasm of the Pancreas Requiring Pancreaticoduodenectomy Without Pancreatojejunostomy: A Case Report and Literature Review.Cureus. 2024 Jul 1;16(7):e63603. doi: 10.7759/cureus.63603. eCollection 2024 Jul. Cureus. 2024. PMID: 39087146 Free PMC article.
-
Prevalence of a BRCA2 Pathogenic Variant in Hereditary-Breast-and-Ovarian-Cancer-Syndrome Families with Increased Risk of Pancreatic Cancer in a Restricted Italian Area.Cancers (Basel). 2023 Apr 3;15(7):2132. doi: 10.3390/cancers15072132. Cancers (Basel). 2023. PMID: 37046793 Free PMC article.
-
Mucinous Cystic Neoplasms in Male Patients: Histopathological and Molecular Diagnoses.Curr Oncol. 2025 Jun 13;32(6):352. doi: 10.3390/curroncol32060352. Curr Oncol. 2025. PMID: 40558295 Free PMC article.
-
Fully automated magnetic resonance imaging-based radiomics analysis for differentiating pancreatic adenosquamous carcinoma from pancreatic ductal adenocarcinoma.Abdom Radiol (NY). 2023 Jun;48(6):2074-2084. doi: 10.1007/s00261-023-03801-8. Epub 2023 Mar 25. Abdom Radiol (NY). 2023. PMID: 36964775
References
Publication types
LinkOut - more resources
Full Text Sources

