Organ-on-a-Chip systems for new drugs development
- PMID: 35299767
- PMCID: PMC8920106
- DOI: 10.5599/admet.942
Organ-on-a-Chip systems for new drugs development
Abstract
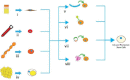
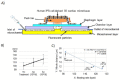
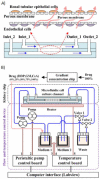
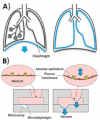
Research on alternatives to the use of animal models and cell cultures has led to the creation of organ-on-a-chip systems, in which organs and their physiological reactions to the presence of external stimuli are simulated. These systems could even replace the use of human beings as subjects for the study of drugs in clinical phases and have an impact on personalized therapies. Organ-on-a-chip technology present higher potential than traditional cell cultures for an appropriate prediction of functional impairments, appearance of adverse effects, the pharmacokinetic and toxicological profile and the efficacy of a drug. This potential is given by the possibility of placing different cell lines in a three-dimensional-arranged polymer piece and simulating and controlling specific conditions. Thus, the normal functioning of an organ, tissue, barrier, or physiological phenomenon can be simulated, as well as the interrelation between different systems. Furthermore, this alternative allows the study of physiological and pathophysiological processes. Its design combines different disciplines such as materials engineering, cell cultures, microfluidics and physiology, among others. This work presents the main considerations of OoC systems, the materials, methods and cell lines used for their design, and the conditions required for their proper functioning. Examples of applications and main challenges for the development of more robust systems are shown. This non-systematic review is intended to be a reference framework that facilitates research focused on the development of new OoC systems, as well as their use as alternatives in pharmacological, pharmacokinetic and toxicological studies.
Keywords: Cell Culture; Drug Discovery; Lab-On-A-Chip Devices; Organoids; Preclinical models; Tissue Engineering.
Copyright © 2021 by the authors.
Conflict of interest statement
Conflict of interest : The authors have no conflicts of interest to declare.
Figures








References
-
- Mittal R., Woo F.W., Castro C.S., Cohen M.A., Karanxha J., Mittal J., Chhibber T., Jhaveri V.M.. Organ-on-chip models: Implications in drug discovery and clinical applications. Journal of Cellular Physiology 234 (2019) 8352-8380. https://doi.org/10.1002/jcp.27729 10.1002/jcp.27729. - DOI - PubMed
-
- Jodat Y.A., Kang M.G., Kiaee K., Kim G.J., Martinez A.F.H., Rosenkranz A., Bae H., Shin S.R.. Human-Derived Organ-on-a-Chip for Personalized Drug Development. Current Pharmaceutical Design 24 (2019) 5471-5486. https://doi.org/10.2174/1381612825666190308150055 10.2174/1381612825666190308150055. - DOI - PMC - PubMed
-
- Lin A., Sved Skottvoll F., Rayner S., Pedersen-Bjergaard S., Sullivan G., Krauss S., Ray Wilson S., Harrison S.. 3D cell culture models and organ-on-a-chip: Meet separation science and mass spectrometry. Electrophoresis 41 (2020) 56-64. https://doi.org/10.1002/elps.201900170 10.1002/elps.201900170. - DOI - PubMed
-
- Kim J.A., Hong S., Rhee W.J.. Microfluidic three-dimensional cell culture of stem cells for high-throughput analysis. World J Stem Cells 11 (2019) 803-816. https://doi.org/10.4252/wjsc.v11.i10.803 10.4252/wjsc.v11.i10.803. - DOI - PMC - PubMed
-
- Miranda C.C., Fernandes T.G., Diogo M.M., Cabral J.M.S.. Towards multi-organoid systems for drug screening applications. Bioengineering 5 (2018) 1-17. https://doi.org/10.3390/bioengineering5030049. 10.3390/bioengineering5030049 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
