Pilot study of autologous fecal microbiota transplants in nursing home residents: Feasibility and safety
- PMID: 35299780
- PMCID: PMC8921299
- DOI: 10.1016/j.conctc.2022.100906
Pilot study of autologous fecal microbiota transplants in nursing home residents: Feasibility and safety
Abstract
Introduction: Antibiotic resistant bacterial infections (ARBIs) are extremely common in nursing home residents. These infections typically occur after a course of antibiotics, which eradicate both pathological and beneficial organisms. The eradication of beneficial organisms likely facilitates subsequent ARBIs. Autologous fecal microbiota transplant (aFMT) has been proposed as a potential treatment to reduce ARBIs in nursing home residents. Our objective was to determine the feasibility and safety of aFMT in a nursing home population.
Methods: Pilot clinical trial. We evaluated feasibility as total number of stool samples collected for aFMT production and safety as the number and relatedness of serious (SAE) and non-serious adverse events (AE).
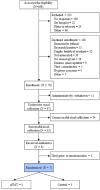
Results: We screened 468 nursing home residents aged ≥18 years for eligibility; 67 enrolled, distributed among three nursing homes. Participants were 62.7% female and 35.8% Black. Mean age was 82.2 ± 8.5 years. Thirty-three participants underwent successful stool collection. Seven participants received antibiotics; four participants underwent aFMT. There were 40 SAEs (17 deaths) and 11 AEs. In the aFMT group, there were 3 SAEs (2 deaths) and 10 AEs. All SAEs and AEs were judged unrelated to the study intervention.
Conclusions: In this pilot study of aFMT in nursing home residents, less than half were able to provide adequate stool samples for aFMT. There were no related SAEs or AEs during the study. In sum, we conclude aFMT has limited feasibility in a nursing home population due to logistic and technical challenges but is likely safe.
Trial registration: ClinicalTrials.gov Identifier: NCT03061097.
Keywords: AE, adverse event; ARBI, antibiotic resistant bacterial infections; Elderly; FMT, fecal microbiota transplant; Fecal transplants; Microbiota; Nursing home; Older adults; SAE, serious adverse event; aFMT, autologous fecal microbiota transplant.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Shrish Budree is a shareholder and employee of Finch Therapeutics. Zain Kassam is a shareholder of Finch Therapeutics.
Similar articles
-
Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain.Gastroenterology. 2021 Jan;160(1):158-173.e10. doi: 10.1053/j.gastro.2020.08.041. Epub 2020 Aug 26. Gastroenterology. 2021. PMID: 32860791 Free PMC article. Clinical Trial.
-
Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients.Nat Commun. 2021 May 25;12(1):3084. doi: 10.1038/s41467-021-23376-6. Nat Commun. 2021. PMID: 34035290 Free PMC article. Clinical Trial.
-
Successful weight regain attenuation by autologous fecal microbiota transplantation is associated with non-core gut microbiota changes during weight loss; randomized controlled trial.Gut Microbes. 2023 Dec;15(2):2264457. doi: 10.1080/19490976.2023.2264457. Epub 2023 Oct 5. Gut Microbes. 2023. PMID: 37796016 Free PMC article. Clinical Trial.
-
Antibiotics for induction and maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Feb 7;2(2):CD012730. doi: 10.1002/14651858.CD012730.pub2. Cochrane Database Syst Rev. 2019. PMID: 30731030 Free PMC article.
-
Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012804. doi: 10.1002/14651858.CD012804.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828765 Free PMC article.
Cited by
-
Mechanisms and Clinical Implications of Human Gut Microbiota-Drug Interactions in the Precision Medicine Era.Biomedicines. 2024 Jan 16;12(1):194. doi: 10.3390/biomedicines12010194. Biomedicines. 2024. PMID: 38255298 Free PMC article. Review.
-
Microbiota Transplantation Among Patients Receiving Long-Term Care: The Sentinel REACT Nonrandomized Clinical Trial.JAMA Netw Open. 2025 Jul 1;8(7):e2522740. doi: 10.1001/jamanetworkopen.2025.22740. JAMA Netw Open. 2025. PMID: 40705333 Free PMC article. Clinical Trial.
-
Safety and tolerability of frozen, capsulized autologous faecal microbiota transplantation. A randomized double blinded phase I clinical trial.PLoS One. 2023 Sep 27;18(9):e0292132. doi: 10.1371/journal.pone.0292132. eCollection 2023. PLoS One. 2023. PMID: 37756322 Free PMC article. Clinical Trial.
-
Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn's Disease Patients Rational and Feasible? Data from a Feasibility Test.Nutrients. 2023 Apr 2;15(7):1742. doi: 10.3390/nu15071742. Nutrients. 2023. PMID: 37049583 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical