miR-218-5p Induces Interleukin-1β and Endovascular Trophoblast Differentiation by Targeting the Transforming Growth Factor β-SMAD2 Pathway
- PMID: 35299960
- PMCID: PMC8920978
- DOI: 10.3389/fendo.2022.842587
miR-218-5p Induces Interleukin-1β and Endovascular Trophoblast Differentiation by Targeting the Transforming Growth Factor β-SMAD2 Pathway
Abstract
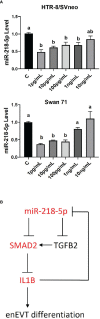
The acquisition of an endovascular trophoblast (enEVT) phenotype is essential for normal placental development and healthy pregnancy. MicroRNAs (miRNAs) are small noncoding RNAs that play critical roles in regulating gene expression. We have recently reported that miR-218-5p promotes enEVT differentiation and spiral artery remodeling in part by targeting transforming growth factor β2 (TGFβ2). We also identified IL1B, which encodes interleukin 1β (IL1β), as one of the most highly upregulated genes by miR-218-5p. In this study, we investigated how miR-218-5p regulates IL1B expression and IL1β secretion and the potential role of IL1β in enEVT differentiation. Using two cell lines derived from extravillous trophoblasts (EVTs), HTR-8/SVneo and Swan 71, we found that stable overexpression of miR-218-5p precursor, mir-218-1, or transient transfection of miR-218-5p mimic, significantly increased IL1B mRNA and IL1β protein levels in cells and conditioned media. We also showed that miR-218-5p directly interacted with SMAD2 3'UTR and reduced SMAD2 at mRNA and protein levels. Knockdown of SMAD2 induced IL1B expression and attenuated the inhibitory effect of TGFβ2 on IL1B expression. On the other hand, overexpression of SMAD2 reduced IL1β levels and blocked the stimulatory effects of miR-218-5p on IL1B expression, trophoblast migration and endothelial-like network formation. In addition, treatment of trophoblasts with IL1β induced the formation of endothelial-like networks and the expression of enEVT markers in a dose-dependent manner. These results suggest that miR-218-5p inhibits the TGFβ/SMAD2 pathway to induce IL1β and enEVT differentiation. Finally, low doses of IL1β also inhibited the expression of miR-218-5p, suggesting the existence of a negative feedback regulatory loop. Taken together, our findings suggest a novel interactive miR-218-5p/TGFβ/SMAD2/IL1β signaling nexus that regulates enEVT differentiation.
Keywords: IL1β; SMAD; TGFβ; endovascular trophoblast; miR-218-5p; placenta.
Copyright © 2022 Shan, Chen, Brkić, Fournier, Ma and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

