Treatment Adherence to Injectable Treatments in Pediatric Growth Hormone Deficiency Compared With Injectable Treatments in Other Chronic Pediatric Conditions: A Systematic Literature Review
- PMID: 35299969
- PMCID: PMC8921265
- DOI: 10.3389/fendo.2022.795224
Treatment Adherence to Injectable Treatments in Pediatric Growth Hormone Deficiency Compared With Injectable Treatments in Other Chronic Pediatric Conditions: A Systematic Literature Review
Abstract
Background: Pediatric patients with growth hormone deficiency (GHD) are currently treated with daily injections of recombinant human growth hormone (rhGH) to promote linear growth and enable attainment of normal adult height. One of the main reasons for suboptimal growth during rhGH therapy is non-adherence to treatment. The objective of this systematic literature review was to examine the recent literature on pediatric adherence to injectable treatments for chronic conditions (focusing on rhGH) to characterize levels of adherence and identify the factors/barriers associated with adherence.
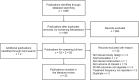
Methods: The Embase and MEDLINE databases (January 2015-October 2020) were searched to identify publications describing studies of pediatric patients (aged ≤17 years) with GHD and other chronic conditions requiring daily or weekly injectable treatments; a similar targeted search of Chinese literature was also performed. Adherence data were extracted from the included studies and summarized. Risk of bias was determined using the Cochrane Risk of Bias tool 2 or the Newcastle-Ottawa Scale.
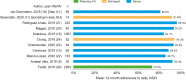
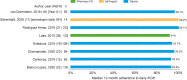
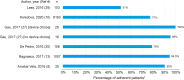
Results: A total of 23 publications were included, with all publications except for one (multiple sclerosis) focused on pediatric GHD studies: there were two clinical trials, 18 observational studies and three survey studies. Study sample sizes ranged from 30 to 13,553 patients (median: 95 patients). The definition of adherence varied between studies and included mean adherence rate, median adherence rate, and the percentage of patients within pre-specified adherence categories. Of the publications assessing adherence to daily rhGH, 11 studies reported 12-month mean adherence rate (range: 73.3%- 95.3%) and eight studies reported median adherence (range: 91%- 99.2%). The barriers to treatment adherence identified included self-administration, increased administration frequency, age (adolescence), longer treatment duration, device design, and insufficient family education, awareness, and/or engagement. Recommendations for increasing adherence included using adherence reminder tools, increasing patient engagement/education, and improving injection device design and drug product.
Conclusions: Adherence to rhGH treatment was high (>80%) for many studies, though comparability between studies was limited given the substantial heterogeneity in the way adherence was defined, measured, and reported. To address this heterogeneity, we recommend standardizing how adherence is defined and reported and encourage the use of standardized study designs and outcome measures.
Keywords: adherence; growth hormone; growth hormone deficiency; injection; pediatric; systematic literature review.
Copyright © 2022 Gomez, Ahmed, Maghnie, Li, Tanaka and Miller.
Conflict of interest statement
RG: employee of and owns shares/options in Pfizer. MM: research support from Pfizer and Merck Serono and consultant for Pfizer, Novo Nordisk, Merck Serono, Ferring, Biomarin, and Ascendis. SFA: unrestricted research and education support from Diurnal, Neurocrine Biosciences, Novo Nordisk; chief investigator for Acerus; and consultant for Sanofi. TT: consultant for JCR pharmaceuticals. BSM: consultant for AbbVie, Ascendis Pharma, BioMarin, Merck Serono, Novo Nordisk, Orchard Therapeutics, Pfizer, Sandoz, Tolmar and Vertice Pharma and research support from Alexion, Abbvie, Amgen, Lumos Pharma, Novo Nordisk, OPKO, and Pfizer. The authors declare that this study received funding from Pfizer. The funder had the following involvement in the study: input into study design, interpretation of data, and preparation of the manuscript. The authors had final authority on all aspects of the manuscript content and development, including on the choice of journal. The reviewer CG declared a past co-authorship with several of the authors (MM and SFA) to the handling editor.
Figures
References
-
- Desrosiers P, O’Brien F, Blethen S. Patient Outcomes in the GHMonitor: The Effect of Delivery Device on Compliance and Growth. Pediatr Endocrinol Rev (2005) 2(Suppl 3):327–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

