Drug Resistance to HIV-1 Integrase Inhibitors Among Treatment-Naive Patients in Beijing, China
- PMID: 35300056
- PMCID: PMC8922317
- DOI: 10.2147/PGPM.S345797
Drug Resistance to HIV-1 Integrase Inhibitors Among Treatment-Naive Patients in Beijing, China
Abstract
Introduction: Integrase strand transfer inhibitors (INSTIs) are important drugs that are currently used as the first line treatment for HIV-1 patients. The aim of this study was to characterize HIV-1 INSTI mutations among ART-naive patients in Beijing from 2019-2021.
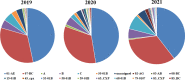
Methods: 865 ART-naive patients were enrolled in this study between January 2019 and June 2021 in Beijing. The amplification of the entire pol gene containing the reverse transcriptase, protease and integrase regions was performed using a validated In-house SBS method. HIV-1 subtypes and circulating recombinant forms (CRFs) were determined using the COMET online tool (http://comet.retrovirology.lu). Stanford HIV-1 drug resistance database (HIVdb version 8.9) was used to analyze the mutations.
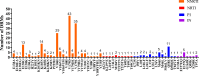
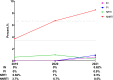
Results: 865 HIV-1 pol sequences were successfully amplified and sequenced. Among them, no major INSTI-related mutations were identified, but 12 polymorphic accessory mutations were found. Two patients have E138A and G163R mutations respectively and both could cause low-level resistance to RAL and EVG. Furthermore, one patient having S230R mutation resulted in low-level resistance to RAL, EVG, DTG and BIC.
Conclusion: The prevalence of INSTIs mutations remains low, which demonstrated that INSTIs have good applicability currently in our city. Nevertheless, it is very important to monitor the INSTI-related mutations in Beijing.
Keywords: Beijing; HIV-1; genotype; integrase strand transfer inhibitor; pre-treatment drug resistance mutation.
© 2022 Yu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Sterne JAC, Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378–384. - PubMed
-
- World Health Organization. HIV Drug Resistance Report 2017. Geneva: World Health Organization; 2017.
-
- World Health Organization. HIV Drug Resistance Report 2019. Geneva: World Health Organization; 2019.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous