Steroid-Quinoline Hybrids for Disruption and Reversion of Protein Aggregation Processes
- PMID: 35300075
- PMCID: PMC8919386
- DOI: 10.1021/acsmedchemlett.1c00604
Steroid-Quinoline Hybrids for Disruption and Reversion of Protein Aggregation Processes
Abstract
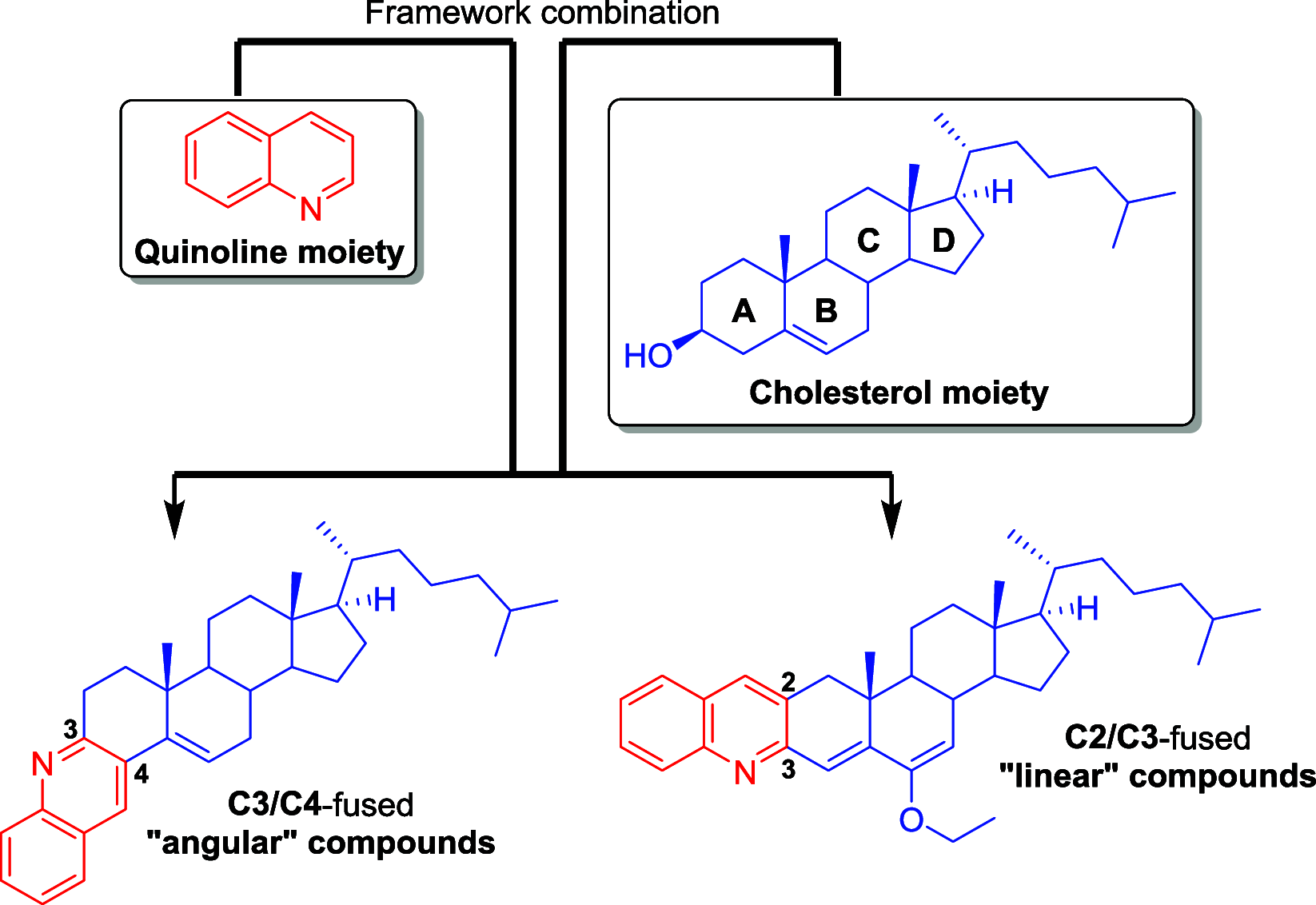
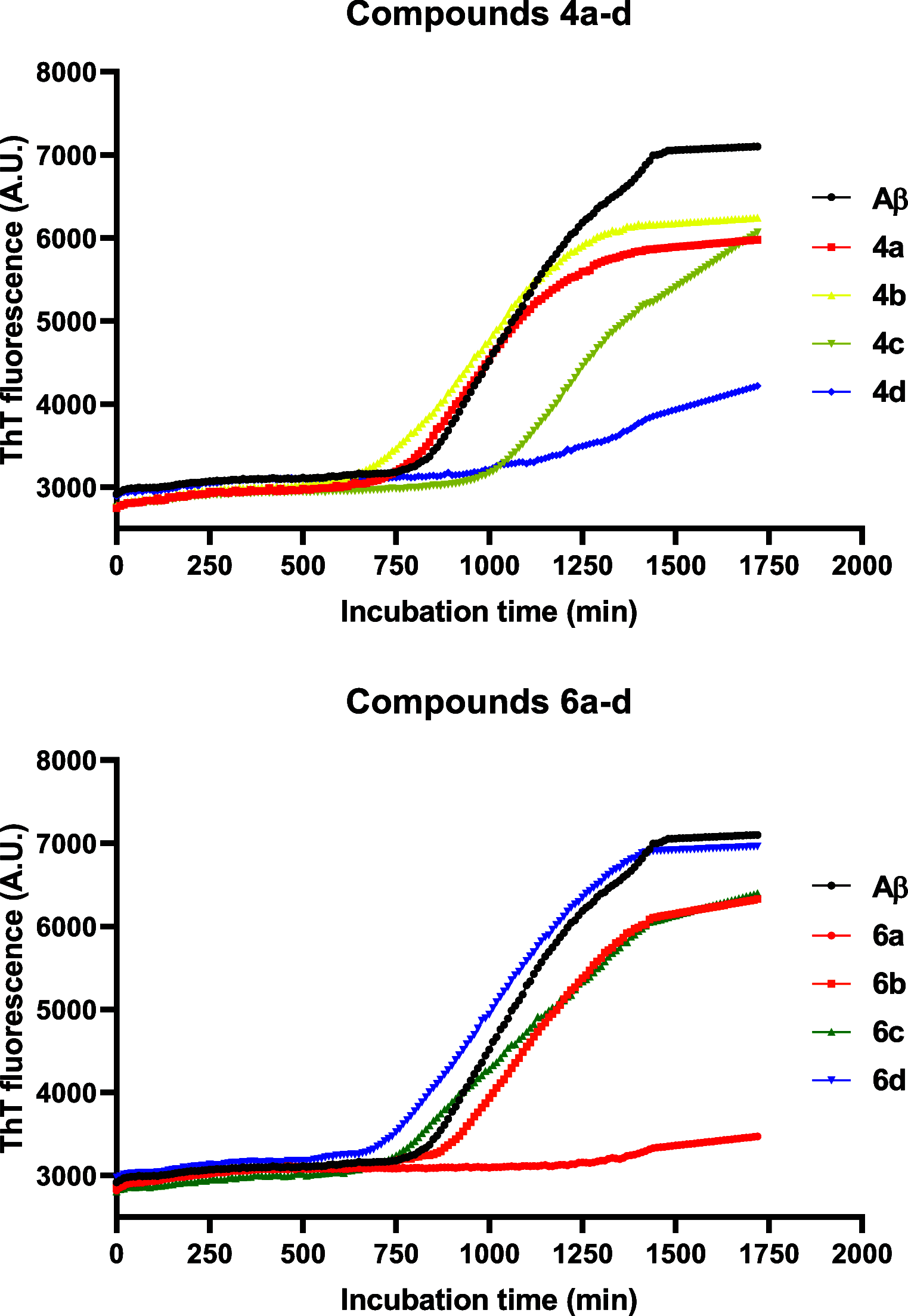
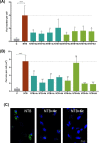
Reversing protein aggregation within cells may be an important tool to fight protein-misfolding disorders such as Alzheimer's, Parkinson's, and cardiovascular diseases. Here we report the design and synthesis of a family of steroid-quinoline hybrid compounds based on the framework combination approach. This set of hybrid compounds effectively inhibited Aβ1-42 self-aggregation in vitro by delaying the exponential growth phase and/or reducing the quantity of fibrils in the steady state. Their disaggregation efficacy was further demonstrated against preaggregated Aβ1-42 peptides in cellular assays upon their endocytosis by neuroblastoma cells, as they reverted both the number and the average area of fibrils back to basal levels. The antiaggregation effect of these hybrids was further tested and demonstrated in a cellular model of general protein aggregation expressing a protein aggregation fluorescent sensor. Together, our results show that the new cholesterol-quinoline hybrids possess wide and marked disaggregation capacities and are therefore promising templates for the development of new drugs to deal with conformational disorders.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
LinkOut - more resources
Full Text Sources

