Development of Myostatin Inhibitory d-Peptides to Enhance the Potency, Increasing Skeletal Muscle Mass in Mice
- PMID: 35300091
- PMCID: PMC8919388
- DOI: 10.1021/acsmedchemlett.1c00705
Development of Myostatin Inhibitory d-Peptides to Enhance the Potency, Increasing Skeletal Muscle Mass in Mice
Abstract
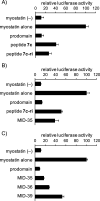
Myostatin is a key negative regulator of skeletal muscle growth, and myostatin inhibitors are attractive tools for the treatment of muscular atrophy. Previously, we reported a series of 14-29-mer peptide myostatin inhibitors, including a potent derivative, MIPE-1686, a 16-mer N-terminal-free l-peptide with three unnatural amino acids and a propensity to form β-sheets. However, the in vivo biological stability of MIPE-1686 is a concern for its development as a drug. In the present study, to develop a more stable myostatin inhibitory d-peptide (MID), we synthesized various retro-inverso versions of a 16-mer peptide. Among these, an arginine-containing derivative, MID-35, shows a potent and equivalent in vitro myostatin inhibitory activity equivalent to that of MIPE-1686 and considerable stability against biodegradation. The in vivo potency of MID-35 to increase the tibialis anterior muscle mass in mice is significantly enhanced over that of MIPE-1686, and MID-35 can serve as a new entity for the prolonged inactivation of myostatin in skeletal muscle.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Enzymatic Stability of Myostatin Inhibitory 16-mer Peptides.Chem Pharm Bull (Tokyo). 2020;68(6):512-515. doi: 10.1248/cpb.c20-00158. Chem Pharm Bull (Tokyo). 2020. PMID: 32475853
-
Design and synthesis of potent myostatin inhibitory cyclic peptides.Bioorg Med Chem. 2019 Apr 1;27(7):1437-1443. doi: 10.1016/j.bmc.2019.02.019. Epub 2019 Feb 10. Bioorg Med Chem. 2019. PMID: 30777663
-
[Medicinal Chemistry Focused on Mid-sized Peptides Derived from Biomolecules].Yakugaku Zasshi. 2019;139(11):1377-1384. doi: 10.1248/yakushi.19-00149. Yakugaku Zasshi. 2019. PMID: 31685733 Review. Japanese.
-
Identification of the minimum region of flatfish myostatin propeptide (Pep45-65) for myostatin inhibition and its potential to enhance muscle growth and performance in animals.PLoS One. 2019 Apr 18;14(4):e0215298. doi: 10.1371/journal.pone.0215298. eCollection 2019. PLoS One. 2019. PMID: 30998775 Free PMC article.
-
Peptide Tool-Driven Functional Elucidation of Biomolecules Related to Endocrine System and Metabolism.Chem Pharm Bull (Tokyo). 2022;70(6):413-419. doi: 10.1248/cpb.c22-00048. Chem Pharm Bull (Tokyo). 2022. PMID: 35650039 Review.
Cited by
-
Age Is Just a Number: Progress and Obstacles in the Discovery of New Candidate Drugs for Sarcopenia.Cells. 2023 Nov 11;12(22):2608. doi: 10.3390/cells12222608. Cells. 2023. PMID: 37998343 Free PMC article. Review.
-
Considerations for the genotoxicity assessment of middle size peptide drugs containing non-canonical amino acid residues.Genes Environ. 2023 Dec 13;45(1):36. doi: 10.1186/s41021-023-00294-1. Genes Environ. 2023. PMID: 38093344 Free PMC article.
-
Inactivation of myostatin by photooxygenation using functionalized d-peptides.RSC Med Chem. 2023 Jan 3;14(2):386-392. doi: 10.1039/d2md00425a. eCollection 2023 Feb 22. RSC Med Chem. 2023. PMID: 36846372 Free PMC article.
-
Targeting Protein-Protein Interfaces with Peptides: The Contribution of Chemical Combinatorial Peptide Library Approaches.Int J Mol Sci. 2023 Apr 25;24(9):7842. doi: 10.3390/ijms24097842. Int J Mol Sci. 2023. PMID: 37175549 Free PMC article. Review.
-
Increasing Skeletal Muscle Mass in Mice by Non-Invasive Intramuscular Delivery of Myostatin Inhibitory Peptide by Iontophoresis.Pharmaceuticals (Basel). 2023 Mar 6;16(3):397. doi: 10.3390/ph16030397. Pharmaceuticals (Basel). 2023. PMID: 36986496 Free PMC article.
References
-
- Ojima C.; Noguchi Y.; Miyamoto T.; Saito Y.; Orihashi H.; Yoshimatsu Y.; Watabe T.; Takayama K.; Hayashi Y.; Itoh F. Peptide-2 from mouse myostatin precursor protein alleviates muscle wasting in cancer-associated cachexia. Cancer Sci. 2020, 111, 2954–2964. 10.1111/cas.14520. - DOI - PMC - PubMed
- Zhou X.; Wang J. L.; Lu J.; Song Y.; Kwak K. S.; Jiao Q.; Rosenfeld R.; Chen Q.; Boone T.; Simonet W. S.; Lacey D. L.; Goldberg A. L.; Han H.Q. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142 (4), 531–543. 10.1016/j.cell.2010.07.011. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

