Can small drugs predict the intrinsic aqueous solubility of 'beyond Rule of 5' big drugs?
- PMID: 35300304
- PMCID: PMC8915605
- DOI: 10.5599/admet.794
Can small drugs predict the intrinsic aqueous solubility of 'beyond Rule of 5' big drugs?
Abstract
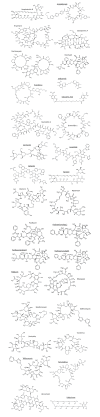
The aim of the study was to explore to what extent small molecules (mostly from the Rule of 5 chemical space) can be used to predict the intrinsic aqueous solubility, S0, of big molecules from beyond the Rule of 5 (bRo5) space. It was demonstrated that the General Solubility Equation (GSE) and the Abraham Solvation Equation (ABSOLV) underpredict solubility in systematic but slightly ways. The Random Forest regression (RFR) method predicts solubility more accurately, albeit in the manner of a 'black box.' It was discovered that the GSE improves considerably in the case of big molecules when the coefficient of the log P term (octanol-water partition coefficient) in the equation is set to -0.4 instead of the traditional -1 value. The traditional GSE underpredicts solubility for molecules with experimental S0 < 50 μM. In contrast, the ABSOLV equation (trained with small molecules) underpredicts the solubility of big molecules in all cases tested. It was found that the errors in the ABSOLV-predicted solubilities of big molecules correlate linearly with the number of rotatable bonds, which suggests that flexibility may be an important factor in differentiating solubility of small from big molecules. Notably, most of the 31 big molecules considered have negative enthalpy of solution: these big molecules become less soluble with increasing temperature, which is compatible with 'molecular chameleon' behavior associated with intramolecular hydrogen bonding. The X-ray structures of many of these molecules reveal void spaces in their crystal lattices large enough to accommodate many water molecules when such solids are in contact with aqueous media. The water sorbed into crystals suspended in aqueous solution may enhance solubility by way of intra-lattice solute-water interactions involving the numerous H-bond acceptors in the big molecules studied. A 'Solubility Enhancement-Big Molecules' index was defined, which embodies many of the above findings.
Keywords: Abraham Solvation Equation (ABSOLV); General Solubility Equation (GSE); Partial Least Squares (PLS); Random Forest regression (RFR); Rule of 5 (Ro5), beyond Ro5 (bRo5); Solubility Enhancement–Big Molecules (SEBM); aqueous intrinsic solubility; intramolecular hydrogen bonding (IMHB).
Copyright © 2020 by the authors.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures







References
-
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 46 (2001) 3–26. - PubMed
-
- Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 46 (2001) 75–87. - PubMed
-
- Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 17 (2012) 486–95. - PubMed
-
- Bergström CAS, Holm R, Jørgensen SA, Andersson SBE, Artursson P, Beato S, et al. Early pharmaceutical profiling to predict oral drug absorption: Current status and unmet needs. Eur J Pharm Sci. 57 (2014) 173–99. - PubMed
-
- Dearden JC. In silico prediction of aqueous solubility. Expert Opin Drug Discov. 1 (2006) 31–52. - PubMed
LinkOut - more resources
Full Text Sources
