Pathways and Mechanisms of Cellular Cholesterol Efflux-Insight From Imaging
- PMID: 35300409
- PMCID: PMC8920967
- DOI: 10.3389/fcell.2022.834408
Pathways and Mechanisms of Cellular Cholesterol Efflux-Insight From Imaging
Abstract
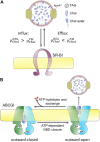
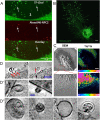
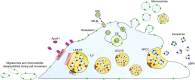
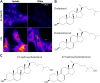
Cholesterol is an essential molecule in cellular membranes, but too much cholesterol can be toxic. Therefore, mammalian cells have developed complex mechanisms to remove excess cholesterol. In this review article, we discuss what is known about such efflux pathways including a discussion of reverse cholesterol transport and formation of high-density lipoprotein, the function of ABC transporters and other sterol efflux proteins, and we highlight their role in human diseases. Attention is paid to the biophysical principles governing efflux of sterols from cells. We also discuss recent evidence for cholesterol efflux by the release of exosomes, microvesicles, and migrasomes. The role of the endo-lysosomal network, lipophagy, and selected lysosomal transporters, such as Niemann Pick type C proteins in cholesterol export from cells is elucidated. Since oxysterols are important regulators of cellular cholesterol efflux, their formation, trafficking, and secretion are described briefly. In addition to discussing results obtained with traditional biochemical methods, focus is on studies that use established and novel bioimaging approaches to obtain insight into cholesterol efflux pathways, including fluorescence and electron microscopy, atomic force microscopy, X-ray tomography as well as mass spectrometry imaging.
Keywords: ABC transporter; HDL; LDL; apoprotein A1 (Apo A1); cholesterol; extracellular vesicles; niemann pick disease; oxysterol.
Copyright © 2022 Juhl and Wüstner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

