Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy Family Members With a Pathogenic NOTCH3 Variant Can Have a Normal Brain Magnetic Resonance Imaging and Skin Biopsy Beyond Age 50 Years
- PMID: 35300531
- PMCID: PMC9126263
- DOI: 10.1161/STROKEAHA.121.036307
Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy Family Members With a Pathogenic NOTCH3 Variant Can Have a Normal Brain Magnetic Resonance Imaging and Skin Biopsy Beyond Age 50 Years
Abstract
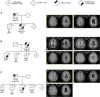
Background: To determine whether extremely mild small vessel disease (SVD) phenotypes can occur in NOTCH3 variant carriers from Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) pedigrees using clinical, genetic, neuroimaging, and skin biopsy findings.
Methods: Individuals from CADASIL pedigrees fulfilling criteria for extremely mild NOTCH3-associated SVD (mSVDNOTCH3) were selected from the cross-sectional Dutch CADASIL cohort (n=200), enrolled between 2017 and 2020. Brain magnetic resonance imaging were quantitatively assessed for SVD imaging markers. Immunohistochemistry and electron microscopy was used to quantitatively assess and compare NOTCH3 ectodomain (NOTCH3ECD) aggregation and granular osmiophilic material deposits in the skin vasculature of mSVDNOTCH3 cases and symptomatic CADASIL patients.
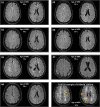
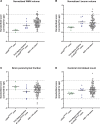
Results: Seven cases were identified that fulfilled the mSVDNOTCH3 criteria, with a mean age of 56.6 years (range, 50-72). All of these individuals harbored a NOTCH3 variant located in one of EGFr domains 7-34 and had a normal brain magnetic resonance imaging, except the oldest individual, aged 72, who had beginning confluence of WMH (Fazekas score 2) and 1 cerebral microbleed. mSVDNOTCH3 cases had very low levels of NOTCH3ECD aggregation in skin vasculature, which was significantly less than in symptomatic EGFr 7-34 CADASIL patients (P=0.01). Six mSVDNOTCH3 cases had absence of granular osmiophilic material deposits.
Conclusions: Our findings demonstrate that extremely mild SVD phenotypes can occur in individuals from CADASIL pedigrees harboring NOTCH3 EGFr 7-34 variants with normal brain magnetic resonance imaging up to age 58 years. Our study has important implications for CADASIL diagnosis, disease prediction, and the counseling of individuals from EGFr 7-34 CADASIL pedigrees.
Keywords: biopsy; brain; capillaries; vascular dementia; white matter.
Figures

Comment in
-
Clinical patterns in CADASIL.J Neurol. 2022 Aug;269(8):4575-4577. doi: 10.1007/s00415-022-11261-1. Epub 2022 Jul 14. J Neurol. 2022. PMID: 35833984 Free PMC article. No abstract available.
References
-
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9 - PubMed
-
- Rutten JW, Hack RJ, Duering M, Gravesteijn G, Dauwerse JG, Overzier M, van den Akker EB, Slagboom E, Holstege H, Nho K, et al. . Broad phenotype of cysteine-altering NOTCH3 variants in UK Biobank: CADASIL to nonpenetrance. Neurology. 2020;95:e1835–e1843. doi: 10.1212/WNL.0000000000010525 - PMC - PubMed
-
- Cho BPH, Nannoni S, Harshfield EL, Tozer D, Gräf S, Bell S, Markus HS. NOTCH3 variants are more common than expected in the general population and associated with stroke and vascular dementia: an analysis of 200 000 participants. J Neurol Neurosurg Psychiatry. 2021;92:694–701. doi: 10.1136/jnnp-2020-325838 - PMC - PubMed
-
- Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(Pt 11):2533–2539. doi: 10.1093/brain/awh282 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

