Multiubiquitination of TRPV4 reduces channel activity independent of surface localization
- PMID: 35300980
- PMCID: PMC9010760
- DOI: 10.1016/j.jbc.2022.101826
Multiubiquitination of TRPV4 reduces channel activity independent of surface localization
Abstract
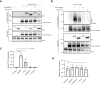
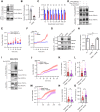
Ubiquitin (Ub)-mediated regulation of plasmalemmal ion channel activity canonically occurs via stimulation of endocytosis. Whether ubiquitination can modulate channel activity by alternative mechanisms remains unknown. Here, we show that the transient receptor potential vanilloid 4 (TRPV4) cation channel is multiubiquitinated within its cytosolic N-terminal and C-terminal intrinsically disordered regions (IDRs). Mutagenizing select lysine residues to block ubiquitination of the N-terminal but not C-terminal IDR resulted in a marked elevation of TRPV4-mediated intracellular calcium influx, without increasing cell surface expression levels. Conversely, enhancing TRPV4 ubiquitination via expression of an E3 Ub ligase reduced TRPV4 channel activity but did not decrease plasma membrane abundance. These results demonstrate Ub-dependent regulation of TRPV4 channel function independent of effects on plasma membrane localization. Consistent with ubiquitination playing a key negative modulatory role of the channel, gain-of-function neuropathy-causing mutations in the TRPV4 gene led to reduced channel ubiquitination in both cellular and Drosophila models of TRPV4 neuropathy, whereas increasing mutant TRPV4 ubiquitination partially suppressed channel overactivity. Together, these data reveal a novel mechanism via which ubiquitination of an intracellular flexible IDR domain modulates ion channel function independently of endocytic trafficking and identify a contributory role for this pathway in the dysregulation of TRPV4 channel activity by neuropathy-causing mutations.
Keywords: Charcot–Marie–Tooth disease; NEDD4; TRPV4; channel activation; ion channel; plasma membrane; ubiquitination.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Kullmann D.M., Hanna M.G. Neurological disorders caused by inherited ion-channel mutations. Lancet Neurol. 2002;1:157–166. - PubMed
-
- Ekberg J., Schuetz F., Boase N.A., Conroy S.-J., Manning J., Kumar S., Poronnik P., Adams D.J. Regulation of the voltage-gated K + channels KCNQ2/3 and KCNQ3/5 by ubiquitination. J. Biol. Chem. 2007;282:12135–12142. - PubMed
-
- Foot N., Henshall T., Kumar S. Ubiquitination and the regulation of membrane proteins. Physiol. Rev. 2016;97:253–281. - PubMed
-
- d’Azzo A., Bongiovanni A., Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–441. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

