Towards a more patient-centered clinical trial process: A systematic review of interventions incorporating health literacy best practices
- PMID: 35301134
- PMCID: PMC9196949
- DOI: 10.1016/j.cct.2022.106733
Towards a more patient-centered clinical trial process: A systematic review of interventions incorporating health literacy best practices
Abstract
Background: A 2019 public workshop convened by the National Academies of Sciences, Engineering and Medicine (NASEM) Roundtable on Health Literacy identified a need to develop evidence-based guidance for best practices for health literacy and patient activation in clinical trials.
Purpose: To identify studies of health literacy interventions within medical care or clinical trial settings that were associated with improved measures of health literacy or patient activation, to help inform best practices in the clinical trial process.
Data sources: Literature searches were conducted in PubMed, the Cumulative Index to Nursing and Allied Health Literature, SCOPUS, Cochrane, and Web of Science from January 2009 to June 2021.
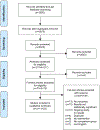
Study selection: Of 3592 records screened, 22 records investigating 27 unique health literacy interventions in randomized controlled studies were included for qualitative synthesis.
Data extraction: Data screening and abstraction were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Data synthesis: Types of health literacy interventions were multimedia or technology-based (11 studies), simplification of written material (six studies) and in-person sessions (five studies). These interventions were applied at various stages in the healthcare and clinical trial process. All studies used unique outcome measures, including patient comprehension, quality of informed consent, and patient activation and engagement.
Conclusions: The findings of our study suggest that best practice guidelines recommend health literacy interventions during the clinical trial process, presentation of information in multiple forms, involvement of patients in information optimization, and improved standardization in health literacy outcome measures.
Keywords: Clinical trial process; Health literacy; Patient-centered; Qualitative synthesis; Systematic literature review.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest
Mehnaz Bader: Employee of Pfizer.
Linda Zheng: No conflicts of interest to declare.
Deepika Rao: No conflicts of interest to declare.
Olayinka Shiyanbola: No conflicts of interest to declare.
Laurie Myers: Employee and stockholder of Merck & Co., Inc.
Terry Davis: No conflicts of interest to declare.
Catina O’Leary: Employee of Health Literacy Media.
Michael McKee: No conflicts of interest to declare.
Michael Wolf: Consultancy from the National Comprehensive Cancer Network, LUTO UK, Sanofi, and Lundbeck; and grants/contract support from Pfizer, Eli Lilly, and Amgen.
Annlouise R. Assaf: Employee of and owns stock in Pfizer.
References
-
- U.S. Department of Health and Human Services. Health Literacy in Healthy People 2030. (last update August 24, 2021). https://health.gov/our-work/national-health-initiatives/healthy-people/h.... Accessed November, 2021.
-
- U.S. Department of Health and Human Services National Institutes of Health. Health Literacy. https://www.nih.gov/institutes-nih/nih-office-director/office-communicat..., 2021. Accessed December, 2021.
-
- Kutner M, Greenberg E, Jin Y, Paulsen C, The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy: U.S Department of Education, National Center for Education Statistics, Washington, DC, 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical