STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice
- PMID: 35301236
- PMCID: PMC8932276
- DOI: 10.1136/jitc-2021-004384
STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice
Abstract
Background: Myeloid-derived suppressor cells (MDSCs) represent a negative prognostic factor in malignant melanoma. These cells are generated under chronic inflammatory conditions typical of cancer. The transcription factor signal transducer and activator of transcription 3 (STAT3) orchestrates MDSC accumulation and acquisition of immunosuppressive properties. Here we studied STAT3 inhibition by Napabucasin as a way to block MDSC accumulation and activity and its potential to treat malignant melanoma.
Methods: In vitro generated murine MDSC and primary MDSC from melanoma-bearing mice were used to investigate the effects of Napabucasin on MDSC in vitro. The RET transgenic mouse model of malignant melanoma was used to examine Napabucasin therapy efficiency and its underlying mechanisms in vivo. Furthermore, STAT3 activation and its correlation with survival were explored in MDSC from 19 patients with malignant melanoma and human in vitro generated monocytic myeloid-derived suppressor cell (M-MDSC) were used to evaluate the effects of Napabucasin.
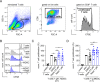
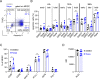
Results: Napabucasin was able to abrogate the capacity of murine MDSC to suppress CD8+ T-cell proliferation. The STAT3 inhibitor induced apoptosis in murine MDSC, significantly increased expression of molecules associated with antigen processing and presentation, as well as slightly decreased expression of immunosuppressive factors on these cells. RET transgenic mice treated with Napabucasin showed prolonged survival accompanied by a strong accumulation of tumor-infiltrating antigen-presenting cells and activation of CD8+ and CD4+ T cells. Interestingly, patients with malignant melanoma with high expression of activated STAT3 in circulating M-MDSC showed significantly worse progression-free survival (PFS) than patients with low levels of activated STAT3. In addition, Napabucasin was able to abrogate suppressive capacity of human in vitro generated M-MDSC.
Conclusion: Our findings demonstrate that STAT3 inhibitor Napabucasin completely abrogated the immunosuppressive capacity of murine MDSC and human M-MDSC and improved melanoma-bearing mouse survival. Moreover, patients with malignant melanoma with high expression levels of activated STAT3 in M-MDSC displayed shorter PFS, indicating its role as a promising therapeutic target in patients with malignant melanoma and a predictive marker for their clinical outcome.
Keywords: immunotherapy; melanoma; myeloid-derived suppressor cells; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
