Cigarette smoking is a secondary cause of folliculin loss
- PMID: 35301243
- PMCID: PMC9612398
- DOI: 10.1136/thoraxjnl-2021-217197
Cigarette smoking is a secondary cause of folliculin loss
Abstract
Background: Birt-Hogg-Dubé syndrome (BHD) is a clinical syndrome manifesting with cystic lung disease and pneumothorax. Features of BHD result from the loss-of-function mutations of the folliculin (FLCN) gene. Chronic obstructive pulmonary disease (COPD), characterised by an irreversible airflow limitation, is primarily caused by cigarette smoking.
Objective: Given that COPD often shares structural features with BHD, we investigated the link between COPD, cigarette smoke (CS) exposure and FLCN expression.
Methods: We measured the expression of FLCN in human COPD lungs and CS-exposed mouse lungs, as well as in CS extract (CSE)-exposed immortalised human airway epithelial cells by immunoblotting.
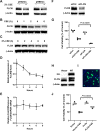
Results: We found that the lung FLCN protein levels in smokers with COPD and CS exposure mice exhibit a marked decrease compared with smokers without COPD and room air exposure mice, respectively. We confirmed CS induced degradation of FLCN in immortalised human bronchial epithelial Beas-2B cells via ubiquitin proteasome system. Further, siRNA targeting FLCN enhanced CSE-induced cytotoxicity. By contrast, FLCN overexpression protected cells from CSE-induced cytotoxicity. We found that FBXO23, the ubiquitin E3 ligase subunit, specifically binds to and targets FLCN for degradation. Inhibition of ATM (ataxia-telangiectasia mutated) attenuated CSE induced FLCN degradation, suggesting a role of ATM in FLCN proteolysis. We further confirmed that the mutant of major FLCN phosphorylation site serine 62A is resistant to CSE-induced degradation and cytotoxicity.
Conclusions: Our study demonstrates that CS exposure is a secondary cause of FLCN deficiency due to the enhanced proteolysis, which promoted airway epithelial cell death.
Keywords: airway epithelium; emphysema; rare lung diseases; tobacco and the lung.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
