Immunological barriers to haematopoietic stem cell gene therapy
- PMID: 35301483
- PMCID: PMC8929255
- DOI: 10.1038/s41577-022-00698-0
Immunological barriers to haematopoietic stem cell gene therapy
Abstract
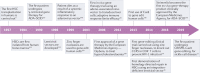
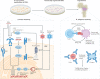
Cell and gene therapies using haematopoietic stem cells (HSCs) epitomize the transformative potential of regenerative medicine. Recent clinical successes for gene therapies involving autologous HSC transplantation (HSCT) demonstrate the potential of genetic engineering in this stem cell type for curing disease. With recent advances in CRISPR gene-editing technologies, methodologies for the ex vivo expansion of HSCs and non-genotoxic conditioning protocols, the range of clinical indications for HSC-based gene therapies is expected to significantly expand. However, substantial immunological challenges need to be overcome. These include pre-existing immunity to gene-therapy reagents, immune responses to neoantigens introduced into HSCs by genetic engineering, and unique challenges associated with next-generation and off-the-shelf HSC products. By synthesizing these factors in this Review, we hope to encourage more research to address the immunological issues associated with current and next-generation HSC-based gene therapies to help realize the full potential of this field.
© 2022. Springer Nature Limited.
Conflict of interest statement
H.N. is a co-founder and shareholder in Megakaryon, Century Therapeutic and Celaid Therapeutics. The other authors declare no competing interests.
Figures


References
-
- Thomas ED, Lochte HL, Jr., Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957;257:491–496. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

