Improved NK Cell Recovery Following Use of PTCy or Treg Expanded Donors in Experimental MHC-Matched Allogeneic HSCT
- PMID: 35302008
- PMCID: PMC9197938
- DOI: 10.1016/j.jtct.2022.03.012
Improved NK Cell Recovery Following Use of PTCy or Treg Expanded Donors in Experimental MHC-Matched Allogeneic HSCT
Abstract
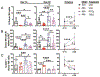
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is complicated by graft- versus-host disease (GVHD), which causes immune dysfunction and further delays immune reconstitution through its effects on primary and secondary lymphoid organs. Treatments to prevent GVHD and improve immune recovery following allo-HSCT are needed. Post-transplantation cyclophosphamide (PTCy) is a well-established and clinically widely used method for GVHD prophylaxis after HLA-matched as well as haploidentical allo-HSCT, as well as a promising strategy in the setting of mismatched unrelated donor allo-HSCT. Recently, regulatory T cells (Tregs), a critical subset for immune homeostasis and tolerance induction, have been evaluated for use as GVHD prophylaxis in experimental models and clinical trials. Natural killer (NK) cells are one of the first lymphoid populations to reconstitute following allo-HSCT and are important mediators of protective immunity against pathogens, and are also critical for limiting post-transplantation relapse of hematologic cancers. Several reports have noted that a delay in NK cell recovery may occur following experimental mouse allo-HSCT as well as after clinical allo-HSCT. Here we examined how 2 treatment strategies, PTCy and donor expanded Tregs (TrED), in experimental MHC-matched allo-HSCT affect NK recovery. Our experiments show that both strategies improved NK cell numbers, with PTCy slightly better than TrED, early after allo-HSCT (1 month) compared with untreated allo-HSCT recipients. Importantly, NK cell IFN-γ production and cytotoxic function, as reflected by CD107 expression as well as in vivo killing of NK-sensitive tumor cells, were improved using either PTCy or TrED versus control allo-HSCT recipients. In conclusion, both prophylactic treatments were found to be beneficial for NK recovery and NK cell function following MHC-matched minor antigen-mismatched experimental allo-HSCT. Improved NK recovery could help provide early immunity toward tumors and pathogens in these transplant recipients.
Keywords: Allogeneic hematopoietic stem cell transplantation; Graft-versus-host disease; IL-2; Natural killer cell; Post-transplantation cyclophosphamide; Regulatory T cell; TL1A.
Copyright © 2022 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosure Statement
RBL is a compensated consultant/advisory board member for and equity holder in Heat Biologics. KVK is an ad hoc consultant for Janssen, Novartis, Genentech/Roche, Kite, Takeda, Iovance, United Healthcare, Avacta Therapeutics and Kiadis. None are directly relevant to the efforts of this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Superior immune reconstitution using Treg-expanded donor cells versus PTCy treatment in preclinical HSCT models.JCI Insight. 2018 Oct 18;3(20):e121717. doi: 10.1172/jci.insight.121717. JCI Insight. 2018. PMID: 30333311 Free PMC article.
-
Increased Infections and Delayed CD4+ T Cell but Faster B Cell Immune Reconstitution after Post-Transplantation Cyclophosphamide Compared to Conventional GVHD Prophylaxis in Allogeneic Transplantation.Transplant Cell Ther. 2021 Nov;27(11):940-948. doi: 10.1016/j.jtct.2021.07.023. Epub 2021 Jul 28. Transplant Cell Ther. 2021. PMID: 34329754
-
Comparison of Regulatory T-Cell Subpopulations in Antithymocytic Globulin Versus Post-Transplant Cyclophosphamide for Preventing Graft-Versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation-A Retrospective Study.Int J Mol Sci. 2025 Mar 11;26(6):2521. doi: 10.3390/ijms26062521. Int J Mol Sci. 2025. PMID: 40141165 Free PMC article.
-
Systematic overview of HLA-matched allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide.Int J Hematol. 2022 Oct;116(4):465-481. doi: 10.1007/s12185-022-03428-3. Epub 2022 Aug 5. Int J Hematol. 2022. PMID: 35930118
-
Graft-versus-tumor effect of post-transplant cyclophosphamide-based allogeneic hematopoietic cell transplantation.Front Immunol. 2024 Jun 6;15:1403936. doi: 10.3389/fimmu.2024.1403936. eCollection 2024. Front Immunol. 2024. PMID: 38903503 Free PMC article. Review.
Cited by
-
Graft-versus-host disease: teaching old drugs new tricks at less cost.Front Immunol. 2023 Aug 3;14:1225748. doi: 10.3389/fimmu.2023.1225748. eCollection 2023. Front Immunol. 2023. PMID: 37600820 Free PMC article. Review.
References
-
- Murphy WJ, Bennett M, Kumar V, Longo DL. Donor-type activated natural killer cells promote marrow engraftment and B cell development during allogeneic bone marrow transplantation. J Immunol. 1992;148:2953–2960. - PubMed
-
- Hu B, He Y, Wu Y, et al. Activated allogeneic NK cells as suppressors of alloreactive responses. Biol Blood Marrow Transplant. 2010;16:772–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

