Persistent cell migration emerges from a coupling between protrusion dynamics and polarized trafficking
- PMID: 35302488
- PMCID: PMC8963884
- DOI: 10.7554/eLife.69229
Persistent cell migration emerges from a coupling between protrusion dynamics and polarized trafficking
Abstract
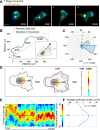
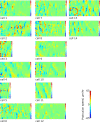
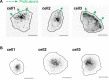
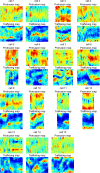
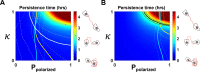
Migrating cells present a variety of paths, from random to highly directional ones. While random movement can be explained by basal intrinsic activity, persistent movement requires stable polarization. Here, we quantitatively address emergence of persistent migration in (hTERT)-immortalizedRPE1 (retinal pigment epithelial) cells over long timescales. By live cell imaging and dynamic micropatterning, we demonstrate that the Nucleus-Golgi axis aligns with direction of migration leading to efficient cell movement. We show that polarized trafficking is directed toward protrusions with a 20-min delay, and that migration becomes random after disrupting internal cell organization. Eventually, we prove that localized optogenetic Cdc42 activation orients the Nucleus-Golgi axis. Our work suggests that polarized trafficking stabilizes the protrusive activity of the cell, while protrusive activity orients this polarity axis, leading to persistent cell migration. Using a minimal physical model, we show that this feedback is sufficient to recapitulate the quantitative properties of cell migration in the timescale of hours.
Keywords: Golgi apparatus; RhoGTPase; cell architecture; cell biology; human; model; optogenetics; persistent migration; physics of living systems; polarity; polarized trafficking; subcellular organization.
© 2022, Vaidžiulytė et al.
Conflict of interest statement
KV, AM, AB, WB, KS, MC No competing interests declared
Figures












References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

