Synthesis and Development of a Novel First-in-Class Cofilin Inhibitor for Neuroinflammation in Hemorrhagic Brain Injury
- PMID: 35302736
- PMCID: PMC9996837
- DOI: 10.1021/acschemneuro.2c00010
Synthesis and Development of a Novel First-in-Class Cofilin Inhibitor for Neuroinflammation in Hemorrhagic Brain Injury
Abstract
Intracerebral hemorrhage (ICH) is devastating among stroke types with high mortality. To date, not a single therapeutic intervention has been successful. Cofilin plays a critical role in inflammation and cell death. In the current study, we embarked on designing and synthesizing a first-in-class small-molecule inhibitor of cofilin to target secondary complications of ICH, mainly neuroinflammation. A series of compounds were synthesized, and two lead compounds SZ-3 and SK-1-32 were selected for further studies. Neuronal and microglial viabilities were assessed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay using neuroblastoma (SHSY-5Y) and human microglial (HMC-3) cell lines, respectively. Lipopolysaccharide (LPS)-induced inflammation in HMC-3 cells was used for neurotoxicity assay. Other assays include nitric oxide (NO) by Griess reagent, cofilin inhibition by F-actin depolymerization, migration by scratch wound assay, tumor necrosis factor (TNF-α) by enzyme-linked immunosorbent assay (ELISA), protease-activated receptor-1 (PAR-1) by immunocytochemistry and Western blotting (WB), and protein expression levels of several proteins by WB. SK-1-32 increased neuronal/microglial survival, reduced NO, and prevented neurotoxicity. However, SZ-3 showed no effect on neuronal/microglial survival but prevented microglia from LPS-induced inflammation by decreasing NO and preventing neurotoxicity. Therefore, we selected SZ-3 for further molecular studies, as it showed potent anti-inflammatory activities. SZ-3 decreased cofilin severing activity, and its treatment of LPS-activated HMC-3 cells attenuated microglial activation and suppressed migration and proliferation. HMC-3 cells subjected to thrombin, as an in vitro model for hemorrhagic stroke, and treated with SZ-3 after 3 h showed significantly decreased NO and TNF-α, significantly increased protein expression of phosphocofilin, and decreased PAR-1. In addition, SZ-3-treated SHSY-5Y showed a significant increase in cell viability by significantly reducing nuclear factor-κ B (NF-κB), caspase-3, and high-temperature requirement (HtrA2). Together, our results support the novel idea of targeting cofilin to counter neuroinflammation during secondary injury following ICH.
Keywords: anti-inflammatory agent; brain injury; cofilin inhibitor; microglial activation; neuroinflammation.
Conflict of interest statement
Figures
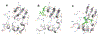
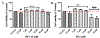
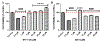
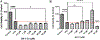

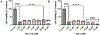

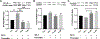

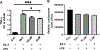

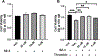



References
-
- Pontaneous S; Ntracerebral I; Emorrhage H; Dnan A; Ureshi IQ; Tanley S; Uhrim T; Oseph J; Roderick PB; Unt HH; Atjer B; Aniel D; Anley FH Review Article Medical Progress 1450·; 2001; Vol. 344.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

