Diffusion and distal linkages govern interchromosomal dynamics during meiotic prophase
- PMID: 35302885
- PMCID: PMC8944930
- DOI: 10.1073/pnas.2115883119
Diffusion and distal linkages govern interchromosomal dynamics during meiotic prophase
Abstract
SignificanceEssential for sexual reproduction, meiosis is a specialized cell division required for the production of haploid gametes. Critical to this process are the pairing, recombination, and segregation of homologous chromosomes (homologs). While pairing and recombination are linked, it is not known how many linkages are sufficient to hold homologs in proximity. Here, we reveal that random diffusion and the placement of a small number of linkages are sufficient to establish the apparent "pairing" of homologs. We also show that colocalization between any two loci is more dynamic than anticipated. Our study provides observations of live interchromosomal dynamics during meiosis and illustrates the power of combining single-cell measurements with theoretical polymer modeling.
Keywords: homologous chromosome pairing; meiosis; polymer physics; tetO/TetR-GFP.
Conflict of interest statement
The authors declare no competing interest.
Figures


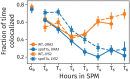
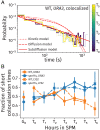
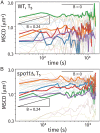
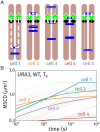
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

