Analysis of accumulated SARS-CoV-2 seroconversion in North Carolina: The COVID-19 Community Research Partnership
- PMID: 35302997
- PMCID: PMC8932589
- DOI: 10.1371/journal.pone.0260574
Analysis of accumulated SARS-CoV-2 seroconversion in North Carolina: The COVID-19 Community Research Partnership
Abstract
Introduction: The COVID-19 Community Research Partnership is a population-based longitudinal syndromic and sero-surveillance study. The study includes over 17,000 participants from six healthcare systems in North Carolina who submitted over 49,000 serology results. The purpose of this study is to use these serology data to estimate the cumulative proportion of the North Carolina population that has either been infected with SARS-CoV-2 or developed a measurable humoral response to vaccination.
Methods: Adult community residents were invited to participate in the study between April 2020 and February 2021. Demographic information was collected and daily symptom screen was completed using a secure, HIPAA-compliant, online portal. A portion of participants were mailed kits containing a lateral flow assay to be used in-home to test for presence of anti-SARS-CoV-2 IgM or IgG antibodies. The cumulative proportion of participants who tested positive at least once during the study was estimated. A standard Cox proportional hazards model was constructed to illustrate the probability of seroconversion over time up to December 20, 2020 (before vaccines available). A separate analysis was performed to describe the influence of vaccines through February 15, 2021.
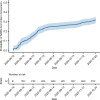
Results: 17,688 participants contributed at least one serology result. 68.7% of the population were female, and 72.2% were between 18 and 59 years of age. The average number of serology results submitted per participant was 3.0 (±1.9). By December 20, 2020, the overall probability of seropositivity in the CCRP population was 32.6%. By February 15, 2021 the probability among healthcare workers and non-healthcare workers was 83% and 49%, respectively. An inflection upward in the probability of seropositivity was demonstrated around the end of December, suggesting an influence of vaccinations, especially for healthcare workers. Among healthcare workers, those in the oldest age category (60+ years) were 38% less likely to have seroconverted by February 15, 2021.
Conclusions: Results of this study suggest more North Carolina residents may have been infected with SARS-CoV-2 than the number of documented cases as determined by positive RNA or antigen tests. The influence of vaccinations on seropositivity among North Carolina residents is also demonstrated. Additional research is needed to fully characterize the impact of seropositivity on immunity and the ultimate course of the pandemic.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Menachemi N, Yiannoutsos CT, Dixon BE, Duszynski TJ, Fadel WF, Wools-Kaloustian KK, et al. Population Point Prevalence of SARS-CoV-2 Infection Based on a Statewide Random Sample—Indiana, April 25–29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(29):960–4. doi: 10.15585/mmwr.mm6929e1 - DOI - PMC - PubMed
-
- Herrington DM, The COVID-19 Community Research Partnership Study Group. Duration of SARS-CoV-2 sero-positivity in a large longitudinal sero-surveillance cohort: the COVID-19 Community Research Partnership. medRxiv 01.27.21250615v1 [Preprint] 2021. [cited 2021 Mar18].Available from: https://www.medrxiv.org/content/10.1101/2021.01.27.21250615v1. - DOI - PMC - PubMed
-
- U.S. Food & Drug Administration, EUA Authorized Serology Test Performance; 2021. [cited 2021 Mar 18]. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em.... [Internet].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous