Physicochemical investigation of a novel curcumin diethyl γ-aminobutyrate, a carbamate ester prodrug of curcumin with enhanced anti-neuroinflammatory activity
- PMID: 35303012
- PMCID: PMC9048745
- DOI: 10.1371/journal.pone.0265689
Physicochemical investigation of a novel curcumin diethyl γ-aminobutyrate, a carbamate ester prodrug of curcumin with enhanced anti-neuroinflammatory activity
Abstract
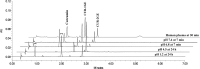
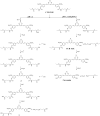
Curcumin is a polyphenol compound that alleviates several neuroinflammation-related diseases including Alzheimer's disease, Parkinson's disease, multiple sclerosis, epilepsy and cerebral injury. However, the therapeutic efficacy of curcumin is limited by its poor physicochemical properties. The present study aimed to develop a new carrier-linked curcumin prodrug, curcumin diethyl γ-aminobutyrate (CUR-2GE), with improved physicochemical and anti-neuroinflammatory properties. CUR-2GE was designed and synthesized by conjugating curcumin with gamma-aminobutyric acid ethyl ester (GE) via a carbamate linkage. The carbamate linkage was selected to increase stability at acidic pH while GE served as a promoiety for lipophilic enhancement. The synthesized CUR-2GE was investigated for solubility, partition coefficient, stability, and bioconversion. The solubility of CUR-2GE was less than 0.05 μg/mL similar to that of curcumin, while the lipophilicity with log P of 3.57 was significantly increased. CUR-2GE was resistant to chemical hydrolysis at acidic pH (pH 1.2 and 4.5) as anticipated but rapidly hydrolyzed at pH 6.8 and 7.4. The incomplete hydrolysis of CUR-2GE was observed in simulated gastrointestinal fluids which liberated the intermediate curcumin monoethyl γ-aminobutyric acid (CUR-1GE) and the parent curcumin. In plasma, CUR-2GE was sequentially converted to CUR-1GE and curcumin within 1 h. In lipopolysaccharide (LPS)-stimulated BV-2 microglial cells, CUR-2GE effectively attenuated the pro-inflammatory mediators by decreasing the secretion of nitric oxide and cytokines (TNF-α and IL-6) to a greater extent than curcumin due to an increase in cellular uptake. Altogether, the newly developed acid-stable CUR-2GE prodrug is a potential pre-clinical and clinical candidate for further evaluation on neuroprotective and anti-neuroinflammatory effects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

