Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study
- PMID: 35303070
- PMCID: PMC9136878
- DOI: 10.1182/blood.2021014162
Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study
Abstract
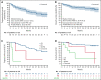
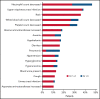
Bruton tyrosine kinase (BTK) inhibitor is an established treatment for relapsed/refractory (R/R) mantle cell lymphoma (MCL). Zanubrutinib, a highly selective BTK inhibitor, is approved for patients with MCL who have received ≥1 prior therapy. We report the long-term safety and efficacy results from the multicenter, open-label, phase 2 registration trial of zanubrutinib. Patients (n = 86) received oral zanubrutinib 160 mg twice daily. The primary endpoint was the overall response rate (ORR), assessed per Lugano 2014. After a median follow-up of 35.3 months, the ORR was 83.7%, with 77.9% achieving complete response (CR); the median duration of response was not reached. Median progression-free survival (PFS) was 33.0 months (95% confidence interval [CI], 19.4-NE). The 36-month PFS and overall survival (OS) rates were 47.6% (95% CI, 36.2-58.1) and 74.8% (95% CI, 63.7-83.0), respectively. The safety profile was largely unchanged with extended follow-up. Most common (≥20%) all-grade adverse events (AEs) were neutrophil count decreased (46.5%), upper respiratory tract infection (38.4%), rash (36.0%), white blood cell count decreased (33.7%), and platelet count decreased (32.6%); most were grade 1/2 events. Most common (≥10%) grade ≥3 AEs were neutrophil count decreased (18.6%) and pneumonia (12.8%). Rates of infection, neutropenia, and bleeding were highest in the first 6 months of therapy and decreased thereafter. No cases of atrial fibrillation/flutter, grade ≥3 cardiac AEs, second primary malignancies, or tumor lysis syndrome were reported. After extended follow-up, zanubrutinib demonstrated durable responses and a favorable safety profile in R/R MCL. The trial is registered at ClinicalTrials.gov as NCT03206970.
© 2022 by The American Society of Hematology.
Figures


Comment in
-
A cauldron of choices.Blood. 2022 May 26;139(21):3103-3104. doi: 10.1182/blood.2022015995. Blood. 2022. PMID: 35616990 No abstract available.
References
-
- Goy A. Mantle cell lymphoma: the changing landscape. Clin Adv Hematol Oncol. 2013;11(suppl 14, pt 9):11-15. - PubMed
-
- Furtado M, Johnson R, Kruger A, Turner D, Rule S. Addition of bortezomib to standard dose chop chemotherapy improves response and survival in relapsed mantle cell lymphoma. Br J Haematol. 2015;168(1):55-62. - PubMed
-
- Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34(12):1386-1394. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

