Impact of phospholipase C β1 in glioblastoma: a study on the main mechanisms of tumor aggressiveness
- PMID: 35303162
- PMCID: PMC8933313
- DOI: 10.1007/s00018-022-04198-1
Impact of phospholipase C β1 in glioblastoma: a study on the main mechanisms of tumor aggressiveness
Abstract
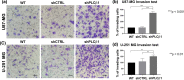
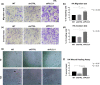
Glioblastoma represents the most lethal brain tumor in adults. Several studies have shown the key role of phospholipase C β1 (PLCβ1) in the regulation of many mechanisms within the central nervous system suggesting PLCβ1 as a novel signature gene in the molecular classification of high-grade gliomas. This study aims to determine the pathological impact of PLCβ1 in glioblastoma, confirming that PLCβ1 gene expression correlates with glioma's grade, and it is lower in 50 glioblastoma samples compared to 20 healthy individuals. PLCβ1 silencing in cell lines and primary astrocytes, leads to increased cell migration and invasion, with the increment of mesenchymal transcription factors and markers, as Slug and N-Cadherin and metalloproteinases. Cell proliferation, through increased Ki-67 expression, and the main survival pathways, as β-catenin, ERK1/2 and Stat3 pathways, are also affected by PLCβ1 silencing. These data suggest a potential role of PLCβ1 in maintaining a normal or less aggressive glioma phenotype.
Keywords: Biomarkers; Brain cancer; Cellular signaling; Glioma; Patients; Phosphoinositides.
© 2022. The Author(s).
Conflict of interest statement
The authors report no competing financial interests.
Figures







Similar articles
-
Phospholipase C Beta 1: a Candidate Signature Gene for Proneural Subtype High-Grade Glioma.Mol Neurobiol. 2016 Nov;53(9):6511-6525. doi: 10.1007/s12035-015-9518-2. Epub 2015 Nov 28. Mol Neurobiol. 2016. PMID: 26614510 Free PMC article.
-
Reduction of phosphoinositide-phospholipase C beta1 methylation predicts the responsiveness to azacitidine in high-risk MDS.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16811-6. doi: 10.1073/pnas.0907109106. Epub 2009 Sep 10. Proc Natl Acad Sci U S A. 2009. PMID: 19805378 Free PMC article.
-
TRIM11 is overexpressed in high-grade gliomas and promotes proliferation, invasion, migration and glial tumor growth.Oncogene. 2013 Oct 17;32(42):5038-47. doi: 10.1038/onc.2012.531. Epub 2012 Nov 26. Oncogene. 2013. PMID: 23178488 Free PMC article.
-
Nuclear Inositide Signaling Via Phospholipase C.J Cell Biochem. 2017 Aug;118(8):1969-1978. doi: 10.1002/jcb.25894. Epub 2017 Apr 25. J Cell Biochem. 2017. PMID: 28106288 Review.
-
Modulation of nuclear PI-PLCbeta1 during cell differentiation.Adv Biol Regul. 2016 Jan;60:1-5. doi: 10.1016/j.jbior.2015.10.008. Epub 2015 Oct 26. Adv Biol Regul. 2016. PMID: 26525203 Review.
Cited by
-
The oily nucleus- role of phospholipids in genome biology: membrane-directed roles and signaling in the nucleoplasm.Cell Mol Life Sci. 2025 Jul 25;82(1):286. doi: 10.1007/s00018-025-05825-3. Cell Mol Life Sci. 2025. PMID: 40711532 Free PMC article. Review.
-
PLCB1 Enhances Cell Migration and Invasion in Gastric Cancer Via Regulating Actin Cytoskeletal Remodeling and Epithelial-Mesenchymal Transition.Biochem Genet. 2023 Dec;61(6):2618-2632. doi: 10.1007/s10528-023-10396-8. Epub 2023 May 19. Biochem Genet. 2023. PMID: 37208557
-
Editorial: Methods in neuro-oncology and neurosurgical oncology.Front Oncol. 2023 Jun 5;13:1213965. doi: 10.3389/fonc.2023.1213965. eCollection 2023. Front Oncol. 2023. PMID: 37342195 Free PMC article. No abstract available.
-
Nuclear Phospholipids and Signaling: An Update of the Story.Cells. 2024 Apr 19;13(8):713. doi: 10.3390/cells13080713. Cells. 2024. PMID: 38667329 Free PMC article. Review.
-
SRPK1 Promotes Glioma Proliferation, Migration, and Invasion through Activation of Wnt/β-Catenin and JAK-2/STAT-3 Signaling Pathways.Biomedicines. 2024 Feb 6;12(2):378. doi: 10.3390/biomedicines12020378. Biomedicines. 2024. PMID: 38397980 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

