(Hetero)aryl-SVI Fluorides: Synthetic Development and Opportunities
- PMID: 35303387
- PMCID: PMC9322316
- DOI: 10.1002/anie.202200904
(Hetero)aryl-SVI Fluorides: Synthetic Development and Opportunities
Abstract
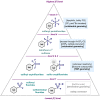
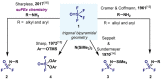
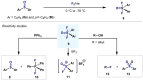
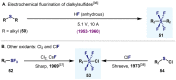
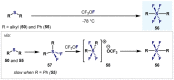
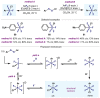
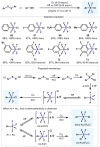
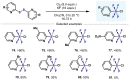
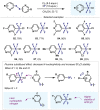
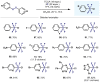
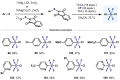
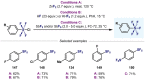
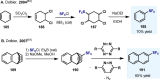
(Hetero)arylsulfur compounds where the S atom is in the oxidation state VI represent a large percentage of the molecular functionalities present in organic chemistry. More specifically, (hetero)aryl-SVI fluorides have recently received enormous attention because of their potential as chemical biology probes, as a result of their reactivity in a simple, modular, and efficient manner. Whereas the synthesis and application of the level 1 fluorination at SVI atoms (sulfonyl and sulfonimidoyl fluorides) have been widely studied and reviewed, the synthetic strategies towards higher levels of fluorination (levels 2 to 5) are somewhat more limited. This Minireview evaluates and summarizes the progress in the synthesis of highly fluorinated aryl-SVI compounds at all levels, discussing synthetic strategies, reactivity, the advantages and disadvantages of the synthetic procedures, the proposed mechanisms, and the potential upcoming opportunities.
Keywords: Fluorine; Pentafluorosulfanyl Arenes; Sulfinyl Trifluorides; Sulfur; Tetrafluorosulfanyl Chlorides.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
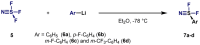
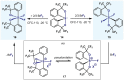
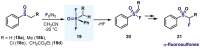
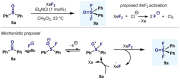
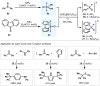


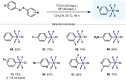

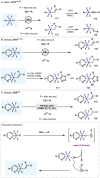


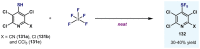


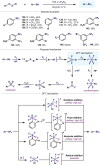
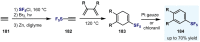
References
-
- For recent examples of aryl sulfonyl chloride reactivity, see
-
- Dubbaka S. R., Vogel P., Angew. Chem. Int. Ed. 2005, 44, 7674–7684; - PubMed
- Angew. Chem. 2005, 117, 7848–7859;
-
- Lu X., Yi Q., Pan X., Wang P., Vessally E. J., J. Sulfur Chem. 2020, 41, 210–228.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

