Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments
- PMID: 35303879
- PMCID: PMC8932066
- DOI: 10.1186/s12943-022-01543-7
Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments
Abstract
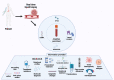
Over the past decade, invasive techniques for diagnosing and monitoring cancers are slowly being replaced by non-invasive methods such as liquid biopsy. Liquid biopsies have drastically revolutionized the field of clinical oncology, offering ease in tumor sampling, continuous monitoring by repeated sampling, devising personalized therapeutic regimens, and screening for therapeutic resistance. Liquid biopsies consist of isolating tumor-derived entities like circulating tumor cells, circulating tumor DNA, tumor extracellular vesicles, etc., present in the body fluids of patients with cancer, followed by an analysis of genomic and proteomic data contained within them. Methods for isolation and analysis of liquid biopsies have rapidly evolved over the past few years as described in the review, thus providing greater details about tumor characteristics such as tumor progression, tumor staging, heterogeneity, gene mutations, and clonal evolution, etc. Liquid biopsies from cancer patients have opened up newer avenues in detection and continuous monitoring, treatment based on precision medicine, and screening of markers for therapeutic resistance. Though the technology of liquid biopsies is still evolving, its non-invasive nature promises to open new eras in clinical oncology. The purpose of this review is to provide an overview of the current methodologies involved in liquid biopsies and their application in isolating tumor markers for detection, prognosis, and monitoring cancer treatment outcomes.
Keywords: Cancer; Circulating tumor DNA; Circulating tumor cells; Liquid biopsy; Non-invasive tumor detection; Precision medicine Cancer diagnosis; Tumor extracellular vesicles.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Hodson R. Precision medicine. Nature. 2016;537:S49–S49. - PubMed
-
- Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–548. - PubMed
-
- Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16:398–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

