Efficacy of a new membrane obturator prosthesis in terms of speech, swallowing, and the quality of life of patients with acquired soft palate defects: study protocol of the VELOMEMBRANE randomized crossover trial
- PMID: 35303932
- PMCID: PMC8931575
- DOI: 10.1186/s13063-022-06163-6
Efficacy of a new membrane obturator prosthesis in terms of speech, swallowing, and the quality of life of patients with acquired soft palate defects: study protocol of the VELOMEMBRANE randomized crossover trial
Abstract
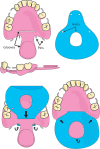
Background: Soft palate defects created during oral cancer surgery may prevent complete palatal closure and trigger palatopharyngeal insufficiency. One current treatment employs a rigid obturator prosthesis; an extension of acrylic resin at the level of the hard palate ensures surface contact with the remaining musculature. Unfortunately, airflow escape often causes hypernasality, compromises speech intelligibility, and creates swallowing problems (including leakage of food and fluid into the nasal airway). We plan to test a new removable denture featuring a thick dental dam that serves as a membrane obturator. The principal objective of the clinical trial is a comparison of speech handicap levels after 1 month in patients with acquired velar insufficiencies who wear either the new device or a conventional, rigid obturator. The secondary objectives are between-device comparisons of the swallowing handicaps and the health-related qualities of life.
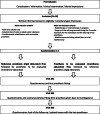
Methods: The VELOMEMBRANE trial is a superiority, open-labeled, two-way, random crossover clinical trial. Adult patients exhibiting velar or palatovelar substance loss after tumor excision and who are indicated for rigid obturator-mediated prosthetic rehabilitation will be recruited in two teaching hospitals in France. Fourteen participants will be randomly allocated to wear both prostheses for 1-month periods in either order. The new membrane obturator is a removable resin prosthesis incorporating a rigid extension that holds a dental dam to restore the soft palate. The primary outcome will be the extent of phonation-related disability (the overall score on the Voice Handicap Index [VHI]). The secondary outcomes will be the Deglutition Handicap Index and health-related quality of life scores of the European Organization for Research and Treatment of Cancer (EORTC).
Discussion: High-quality evidence will be provided to document the utility of a new medical device that may greatly improve the management and quality of life of patients with acquired velar insufficiency.
Trial registration: ClinicalTrials.gov NCT04009811 . Registered on 4 July 2019.
Keywords: Deglutition disorder; Maxillofacial prosthesis; Mouth neoplasm; Palatal obturator; Prosthodontics; Randomized controlled trial; Speech disorder; Velopharyngeal insufficiency.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Defossez G, Le Guyader-Peyrou S, Uhry Z, Grosclaude P, Colonna M, Dantony E, et al. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Tumeurs Solides. 2019;1:372.
-
- Jamerson RE, White JA. Carcinoma of the soft palate and uvula. J La State Med Soc. 1991;143(2):7–9. - PubMed
-
- Schernberg A, Canova C, Blanchard P, Gorphe P, Breuskin I, Mirghani H, Moya-Plana A, Janot F, Bidault F, Chargari C, Bellefqih S, Ruffier A, Even C, Nguyen F, Temam S, Tao Y. Prognostic factors in patients with soft palate squamous cell carcinoma. Head Neck. 2019;41(5):1441–1449. doi: 10.1002/hed.25598. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

