Distinguishing immune activation and inflammatory signatures of multisystem inflammatory syndrome in children (MIS-C) versus hemophagocytic lymphohistiocytosis (HLH)
- PMID: 35304157
- PMCID: PMC8923010
- DOI: 10.1016/j.jaci.2022.02.028
Distinguishing immune activation and inflammatory signatures of multisystem inflammatory syndrome in children (MIS-C) versus hemophagocytic lymphohistiocytosis (HLH)
Abstract
Background: Multisystem inflammatory syndrome in children (MIS-C) is a potentially life-threatening sequela of severe acute respiratory syndrome coronavirus 2 infection characterized by hyperinflammation and multiorgan dysfunction. Although hyperinflammation is a prominent manifestation of MIS-C, there is limited understanding of how the inflammatory state of MIS-C differs from that of well-characterized hyperinflammatory syndromes such as hemophagocytic lymphohistiocytosis (HLH).
Objectives: We sought to compare the qualitative and quantitative inflammatory profile differences between patients with MIS-C, coronavirus disease 2019, and HLH.
Methods: Clinical data abstraction from patient charts, T-cell immunophenotyping, and multiplex cytokine and chemokine profiling were performed for patients with MIS-C, patients with coronavirus disease 2019, and patients with HLH.
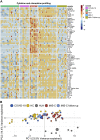
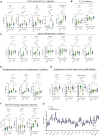
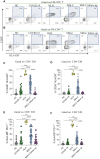
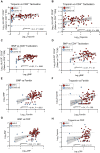
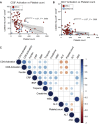
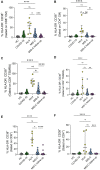
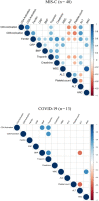
Results: We found that both patients with MIS-C and patients with HLH showed robust T-cell activation, markers of senescence, and exhaustion along with elevated TH1 and proinflammatory cytokines such as IFN-γ, C-X-C motif chemokine ligand 9, and C-X-C motif chemokine ligand 10. In comparison, the amplitude of T-cell activation and the levels of cytokines/chemokines were higher in patients with HLH when compared with patients with MIS-C. Distinguishing inflammatory features of MIS-C included elevation in TH2 inflammatory cytokines such as IL-4 and IL-13 and cytokine mediators of angiogenesis, vascular injury, and tissue repair such as vascular endothelial growth factor A and platelet-derived growth factor. Immune activation and hypercytokinemia in MIS-C resolved at follow-up. In addition, when these immune parameters were correlated with clinical parameters, CD8+ T-cell activation correlated with cardiac dysfunction parameters such as B-type natriuretic peptide and troponin and inversely correlated with platelet count.
Conclusions: Overall, this study characterizes unique and overlapping immunologic features that help to define the hyperinflammation associated with MIS-C versus HLH.
Keywords: COVID-19; HLH; MIS-C; T-cell activation; cardiac dysfunction; hyperinflammation.
Copyright © 2022 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures











References
-
- Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020;109:1082–1083. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

