Signaling sphingolipids are biomarkers for atopic dermatitis prone to disseminated viral infections
- PMID: 35304160
- PMCID: PMC9463085
- DOI: 10.1016/j.jaci.2022.02.027
Signaling sphingolipids are biomarkers for atopic dermatitis prone to disseminated viral infections
Abstract
Background: Life-threatening viral diseases such as eczema herpeticum (EH) and eczema vaccinatum (EV) occur in <5% of individuals with atopic dermatitis (AD). The diagnosis of AD, however, excludes all individuals with AD from smallpox vaccination.
Objectives: We sought to identify circulatory and skin lipid biomarkers associated with EH and EV.
Methods: Stratum corneum and plasma samples from 15 subjects with AD and a history of EH, 13 age- and gender-matched subjects with AD and without EH history, and 13 healthy nonatopic (NA) controls were analyzed by liquid chromatography tandem mass spectrometry for sphingolipid content. Sphingosine-1-phosphate (S1P) and ceramide levels were validated in plasma samples from the Atopic Dermatitis Vaccinia Network/Atopic Dermatitis Research Network repository (12 NA, 12 AD, 23 EH) and plasma from 7 subjects with EV and 7 matched subjects with AD. S1P lyase was downregulated in human primary keratinocytes to evaluate its effect on herpes simplex virus 1 (HSV-1) replication in vitro.
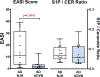
Results: The stratum corneum of patients with EH demonstrated significantly higher levels of free sphingoid bases than those in patients who were NA, indicating enhanced sphingolipid turnover in keratinocytes (P < .05). Plasma from 2 independent cohorts of patients with EH had a significantly increased S1P/ceramide ratio in subjects with EH versus those with AD and or who were NA (P < .01). The S1P level in plasma from subjects with EV was twice the level in plasma from subjects with AD (mean = 1,533 vs 732 pmol/mL; P < .001). Downregulation of S1P lyase expression with silencing RNA led to an increased S1P level and doubled HSV-1 titer in keratinocytes.
Conclusions: Our data point to long-term abnormalities in the S1P signaling system as a biomarker for previous disseminated viral diseases and a potential treatment target in recurring infections.
Keywords: Eczema vaccinatum; S1P lyase; S1P/ceramide ratio; ceramide; eczema herpeticum; human primary keratinocytes; plasma; sphingosine-1-phosphate; stratum corneum.
Copyright © 2022 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

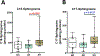


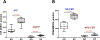
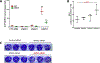
Comment in
-
Sphingolipids in viral skin superinfection: Friend or foe?J Allergy Clin Immunol. 2023 Jan;151(1):108-109. doi: 10.1016/j.jaci.2022.09.031. Epub 2022 Oct 12. J Allergy Clin Immunol. 2023. PMID: 36241047 No abstract available.
References
-
- Bieber T, D’Erme AM, Akdis CA, Traidl-Hoffmann C, Lauener R, Schappi G, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: where are we, and where should we go? J Allergy Clin Immunol 2017;139:S58–S64. - PubMed
-
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020;396:345–60. - PubMed
-
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis 2012;54:832–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

