Amantadine delayed release/extended release capsules significantly reduce OFF time in Parkinson's disease
- PMID: 35304480
- PMCID: PMC8933492
- DOI: 10.1038/s41531-022-00291-1
Amantadine delayed release/extended release capsules significantly reduce OFF time in Parkinson's disease
Abstract
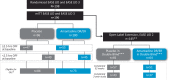
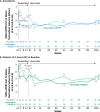
Maintaining consistent levodopa benefits while simultaneously controlling dyskinesia can be difficult. Recently, an amantadine delayed release/extended release (DR/ER) formulation (Gocovri®) indicated for dyskinesia received additional FDA approval as an adjunct to levodopa for the treatment of OFF episodes. We evaluated OFF time reductions with amantadine-DR/ER in a pooled analysis of two phase III amantadine-DR/ER trials (NCT02136914, NCT02274766) followed by a 2-year open-label extension trial (NCT02202551). OFF outcomes were analyzed for the mITT population, as well as stratified by baseline OFF time of ≥2.5 h/day or <2.5 h/day. At Week 12, mean placebo-subtracted treatment difference in OFF time was -1.00 [-1.57, -0.44] h in the mITT population (n = 196), -1.2 [-2.08, -0.32] h in the ≥2.5 h subgroup (n = 102) and -0.77 [-1.49, -0.06] in the <2.5 h subgroup (n = 94). Amantadine-DR/ER-treated participants showed reduced MDS-UPDRS Part IV motor fluctuation subscores by week 2 that were maintained below baseline to Week 100.
© 2022. The Author(s).
Conflict of interest statement
Robert A Hauser was an investigator in the individual studies and reports personal fees from Adamas Pharmaceuticals, Inc. for participating as a Steering Committee member. Judy Lytle and Andrea Formella are employed by Adamas Pharmaceuticals, Inc. Caroline Tanner was an investigator in the individual studies and reports personal fees from Adamas Pharmaceuticals, Inc.
Figures

Similar articles
-
Effects of Gocovri (Amantadine) Extended Release Capsules on Non-Motor Symptoms in Patients with Parkinson's Disease and Dyskinesia.Neurol Ther. 2021 Jun;10(1):307-320. doi: 10.1007/s40120-021-00246-3. Epub 2021 Apr 17. Neurol Ther. 2021. PMID: 33864229 Free PMC article.
-
Effects of Gocovri (Amantadine) Extended-Release Capsules on Motor Aspects of Experiences of Daily Living in People with Parkinson's Disease and Dyskinesia.Neurol Ther. 2021 Dec;10(2):739-751. doi: 10.1007/s40120-021-00256-1. Epub 2021 May 22. Neurol Ther. 2021. PMID: 34024025 Free PMC article.
-
EASE LID 2: A 2-Year Open-Label Trial of Gocovri (Amantadine) Extended Release for Dyskinesia in Parkinson's Disease.J Parkinsons Dis. 2020;10(2):543-558. doi: 10.3233/JPD-191841. J Parkinsons Dis. 2020. PMID: 31929122 Free PMC article. Clinical Trial.
-
Potential utility of amantadine DR/ER in persons with Parkinson's disease meeting 5-2-1 criteria for device aided therapy.Clin Park Relat Disord. 2021 Dec 8;6:100123. doi: 10.1016/j.prdoa.2021.100123. eCollection 2022. Clin Park Relat Disord. 2021. PMID: 35059622 Free PMC article. Review.
-
Amantadine Extended-Release (GOCOVRI™): A Review in Levodopa-Induced Dyskinesia in Parkinson's Disease.CNS Drugs. 2018 Aug;32(8):797-806. doi: 10.1007/s40263-018-0552-2. CNS Drugs. 2018. PMID: 30088203 Review.
Cited by
-
Future Directions for Developing Non-dopaminergic Strategies for the Treatment of Parkinson's Disease.Curr Neuropharmacol. 2024;22(10):1606-1620. doi: 10.2174/1570159X21666230731110709. Curr Neuropharmacol. 2024. PMID: 37526188 Free PMC article. Review.
-
Moving to a non-dopaminergic approach for the treatment of OFF fluctuations in Parkinson's disease.Clin Park Relat Disord. 2025 Jan 27;12:100303. doi: 10.1016/j.prdoa.2025.100303. eCollection 2025. Clin Park Relat Disord. 2025. PMID: 39968317 Free PMC article. Review.
-
Efficacy and safety of oral amantadine in Parkinson's disease with dyskinesia and motor fluctuations: a systematic review and meta-analysis of randomised controlled trials.BMJ Neurol Open. 2025 Jun 15;7(1):e001115. doi: 10.1136/bmjno-2025-001115. eCollection 2025. BMJ Neurol Open. 2025. PMID: 40756069 Free PMC article.
-
Why do 'OFF' periods still occur during continuous drug delivery in Parkinson's disease?Transl Neurodegener. 2022 Oct 13;11(1):43. doi: 10.1186/s40035-022-00317-x. Transl Neurodegener. 2022. PMID: 36229860 Free PMC article. Review.
-
Istradefylline for OFF Episodes in Parkinson's Disease: A US Perspective of Common Clinical Scenarios.Degener Neurol Neuromuscul Dis. 2022 Jul 23;12:97-109. doi: 10.2147/DNND.S245197. eCollection 2022. Degener Neurol Neuromuscul Dis. 2022. PMID: 35910426 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

