Experimental and theoretical study on the corrosion inhibition of mild steel by nonanedioic acid derivative in hydrochloric acid solution
- PMID: 35304485
- PMCID: PMC8933592
- DOI: 10.1038/s41598-022-08146-8
Experimental and theoretical study on the corrosion inhibition of mild steel by nonanedioic acid derivative in hydrochloric acid solution
Abstract
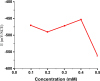
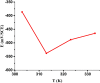
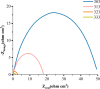
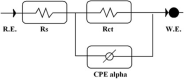
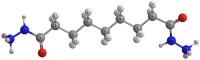
The corrosion performance of mild steel (MS) in 1M HCl solution was examined by weight loss (WL), potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), electrochemical frequency modulation (EFM), and open circuit potential (OCP) measurements in the absence and presence of nonanedihydrazide. PDP measurements indicated that nonanedihydrazide acts as a mixed inhibitor due to its adsorption on the MS surface, exhibiting an inhibition efficiency of more than 97%. The surface morphology investigation of the protective layer on the MS surface confirmed that adsorption of nonanedihydrazide molecules occurred via chemical adsorption following Langmuir's isotherm model. The effect of temperature on the corrosion performance in the presence of nonanedihydrazide was investigated in the range of 303-333 K, showing that the inhibition efficiency increased with an increase in the inhibitor concentration and decreased with an increase in temperature. A new green corrosion inhibitor was synthesised and theoretical computations were conducted to completely understand the inhibition mechanism. Nonanedihydrazide molecules were investigated by DFT (density functional theory) using the B3LYP functional to evaluate the relationship of corrosion inhibition performance and the molecular structure. The computed theoretical parameters presented significant support for understanding the inhibitive mechanism revealed by the inhibitory molecules and are in good agreement with WL, PDP, EIS, (EFM), and OCP results.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












References
-
- Kaczerewska O, Leiva-Garcia R, Akid R, Brycki B. Efficiency of cationic gemini surfactants with 3-azamethylpentamethylene spacer as corrosion inhibitors for stainless steel in hydrochloric acid. J. Mol. Liq. 2017;247:6–13. doi: 10.1016/j.molliq.2017.09.103. - DOI
-
- Goyal M, Kumar S, Bahadur I, Verma C, Ebenso EE. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: a review. J. Mol. Liq. 2018;256:565–573. doi: 10.1016/j.molliq.2018.02.045. - DOI
-
- Li, H. Qiang, Y. Zhao, W. Zhang, S. 2-Mercaptobenzimidazole-inbuilt metal-organic-frameworks modified graphene oxide towards intelligent and excellent anti-corrosion coating, Corrosion Science 191 (2021) 109715
LinkOut - more resources
Full Text Sources

