Modeling absolute zone size in retinopathy of prematurity in relation to axial length
- PMID: 35304549
- PMCID: PMC8933429
- DOI: 10.1038/s41598-022-08680-5
Modeling absolute zone size in retinopathy of prematurity in relation to axial length
Abstract
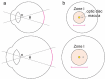
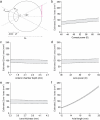
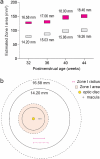
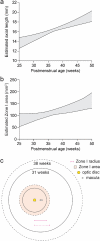
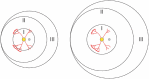
Treatment outcomes in retinopathy of prematurity (ROP) are closely correlated with the location (i.e. zone) of disease, with more posterior zones having poorer outcomes. The most posterior zone, Zone I, is defined as a circle centered on the optic nerve with radius twice the distance from nerve to fovea, or subtending an angle of 30 degrees. Because the eye enlarges and undergoes refractive changes during the period of ROP screening, the absolute area of Zone I according to these definitions may likewise change. It is possible that these differences may confound accurate assessment of risk in patients with ROP. In this study, we estimated the area of Zone I in relation to different ocular parameters to determine how variability in the size and refractive power of the eye may affect zoning. Using Gaussian optics, a model was constructed to calculate the absolute area of Zone I as a function of corneal power, anterior chamber depth, lens power, lens thickness, and axial length (AL), with Zone I defined as a circle with radius set by a 30-degree visual angle. Our model predicted Zone I area to be most sensitive to changes in AL; for example, an increase of AL from 14.20 to 16.58 mm at postmenstrual age 32 weeks was calculated to expand the area of Zone I by up to 72%. These findings motivate several hypotheses which upon future testing may help optimize treatment decisions for ROP.
© 2022. The Author(s).
Conflict of interest statement
S.K.W., None; E.K., Genentech (C), RetiHealth Inc (C,E);, M.Z., None; M.H.J., None; A.A., None; N.F.C, Genentech (E); J.K., Pr3vent INC (F,CEO); D.M.M, 1-800Contacts (BOD,E), Akceso Advisors AG (C), Akebia (SAB), Alcon (C), Aldeyra Therapeutics (PI), Alexion (C), Allegro (SAB), Apellis (PI), Bayer Pharma AG (SC,C), CMEOutfitters.com (C), Congruence Medical Solutions (C), dSentz, Inc. (F,E,BOD), Grand Legend Technology, LTD (E), Iconic Therapeutics (SC), Irenix (SAB), Linc (F,E,BOD), M3 Global Research (C), Northwell Health (C), Novartis Pharmaceuticals (DMC, C), Ocular Surgery News (C), Pr3vent INC (F,E,BOD), Praxis UNS, Inc. (C), Prime Medical Education (C), Promisight, Inc (F,E,BOD), Pykus (E,SAB), Regeneron (SC,PI), Retina Technologies LLC (SAB,C,E), Retina Today/Pentavision (C), Shapiro Law Group (C), SLACK, Inc (C), Versl, Inc. (F,E), Vindico (C), Visunex (E,SAB).
Figures
References
-
- Good WV, et al. The incidence and course of retinopathy of prematurity: Findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116:15–23. - PubMed
-
- Azuma N, et al. Early vitreous surgery for aggressive posterior retinopathy of prematurity. Am. J. Ophthalmol. 2006;142:636–643. - PubMed
-
- Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology. 1993;100:238–244. - PubMed
-
- Steinkuller PG, et al. Childhood blindness. J. AAPOS. 1999;3:26–32. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

