Cell Death and the p53 Enigma During Mammalian Embryonic Development
- PMID: 35304609
- PMCID: PMC9199838
- DOI: 10.1093/stmcls/sxac003
Cell Death and the p53 Enigma During Mammalian Embryonic Development
Abstract
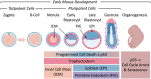
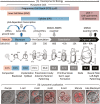
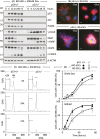
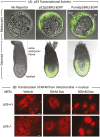
Twelve forms of programmed cell death (PCD) have been described in mammalian cells, but which of them occurs during embryonic development and the role played by the p53 transcription factor and tumor suppressor remains enigmatic. Although p53 is not required for mouse embryonic development, some studies conclude that PCD in pluripotent embryonic stem cells from mice (mESCs) or humans (hESCs) is p53-dependent whereas others conclude that it is not. Given the importance of pluripotent stem cells as models of embryonic development and their applications in regenerative medicine, resolving this enigma is essential. This review reconciles contradictory results based on the facts that p53 cannot induce lethality in mice until gastrulation and that experimental conditions could account for differences in results with ESCs. Consequently, activation of the G2-checkpoint in mouse ESCs is p53-independent and generally, if not always, results in noncanonical apoptosis. Once initiated, PCD occurs at equivalent rates and to equivalent extents regardless of the presence or absence of p53. However, depending on experimental conditions, p53 can accelerate initiation of PCD in ESCs and late-stage blastocysts. In contrast, DNA damage following differentiation of ESCs in vitro or formation of embryonic fibroblasts in vivo induces p53-dependent cell cycle arrest and senescence.
Keywords: apoptosis; cell cycle; differentiation; embryo; p53; pluripotent; programmed cell death; stem cells.
Published by Oxford University Press 2022.
Figures
References
-
- Galluzzi L, Bravo-San Pedro JM, Vitale I, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22(1):58-73. https://doi.org/10.1038/cdd.2014.137 - DOI - PMC - PubMed
-
- Wyllie AH, Kerr JF, Currie AR.. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251-306. https://doi.org/10.1016/s0074-7696(08)62312-8 - DOI - PubMed
-
- Hardy K, Handyside AH, Winston RM.. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development. 1989;107(3):597-604. - PubMed
-
- Fuchs Y, Steller H.. Programmed cell death in animal development and disease. Cell. 2011;147(4):742-758. https://doi.org/10.1016/j.cell.2011.10.033 - DOI - PMC - PubMed
-
- Munoz-Espin D, Canamero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155(5):1104-1118. https://doi.org/10.1016/j.cell.2013.10.019 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

