ChAdOx1-S adenoviral vector vaccine applied intranasally elicits superior mucosal immunity compared to the intramuscular route of vaccination
- PMID: 35304741
- PMCID: PMC9087383
- DOI: 10.1002/eji.202249823
ChAdOx1-S adenoviral vector vaccine applied intranasally elicits superior mucosal immunity compared to the intramuscular route of vaccination
Abstract
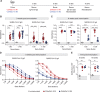
COVID-19 vaccines prevent severe forms of the disease, but do not warrant complete protection against breakthrough infections. This could be due to suboptimal mucosal immunity at the site of virus entry, given that all currently approved vaccines are administered via the intramuscular route. In this study, we assessed humoral and cellular immune responses in BALB/c mice after intranasal and intramuscular immunization with adenoviral vector ChAdOx1-S expressing full-length Spike protein of SARS-CoV-2. We showed that both routes of vaccination induced a potent IgG antibody response, as well as robust neutralizing capacity, but intranasal vaccination elicited a superior IgA antibody titer in the sera and in the respiratory mucosa. Bronchoalveolar lavage from intranasally immunized mice efficiently neutralized SARS-CoV-2, which has not been the case in intramuscularly immunized group. Moreover, substantially higher percentages of epitope-specific CD8 T cells exhibiting a tissue resident phenotype were found in the lungs of intranasally immunized animals. Finally, both intranasal and intramuscular vaccination with ChAdOx1-S efficiently protected the mice after the challenge with recombinant herpesvirus expressing the Spike protein. Our results demonstrate that intranasal application of adenoviral vector ChAdOx1-S induces superior mucosal immunity and therefore could be a promising strategy for putting the COVID-19 pandemic under control.
Keywords: ChAdOx1-S; SARS-CoV-2; mucosal immunity; vaccination; vaccine vectors.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures


References
-
- World Health Organization . WHO Coronavirus (COVID‐19) Dashboard. (2022). Available at: https://covid19.who.int/ (Accessed: 12th January 2022).
-
- Piccoli, L. , Park, Y. J. , Tortorici, M. A. , Czudnochowski, N. , Walls, A. C. , Beltramello, M. , Silacci‐Fregni, C. et al., Mapping neutralizing and immunodominant sites on the SARS‐CoV‐2 spike receptor‐binding domain by structure‐guided high‐resolution serology. Cell 2020. 183: 1024–1042, e1021. - PMC - PubMed
-
- Voysey, M. , Clemens, S. A. C. , Madhi, S. A. , Weckx, L. Y. , Folegatti, P. M. , Aley, P. K. , Angus, B. et al., Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021. 397: 99–111. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

