(+)-Catharanthine potentiates the GABAA receptor by binding to a transmembrane site at the β(+)/α(-) interface near the TM2-TM3 loop
- PMID: 35304861
- PMCID: PMC9178925
- DOI: 10.1016/j.bcp.2022.114993
(+)-Catharanthine potentiates the GABAA receptor by binding to a transmembrane site at the β(+)/α(-) interface near the TM2-TM3 loop
Abstract
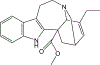
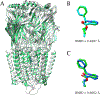
(+)-Catharanthine, a coronaridine congener, potentiates the γ-aminobutyric acid type A receptor (GABAAR) and induces sedation through a non-benzodiazepine mechanism, but the specific site of action and intrinsic mechanism have not beendefined. Here, we describe GABAAR subtype selectivity and location of the putative binding site for (+)-catharanthine using electrophysiological, site-directed mutagenesis, functional competition, and molecular docking experiments. Electrophysiological and in silico experiments showed that (+)-catharanthine potentiates the responses to low, subsaturating GABA at β2/3-containing GABAARs 2.4-3.5 times more efficaciously than at β1-containing GABAARs. The activity of (+)-catharanthine is reduced by the β2(N265S) mutation that decreases GABAAR potentiation by loreclezole, but not by the β3(M286C) or α1(Q241L) mutations that reduce receptor potentiation by R(+)-etomidate or neurosteroids, respectively. Competitive functional experiments indicated that the binding site for (+)-catharanthine overlaps that for loreclezole, but not those for R(+)-etomidate or potentiating neurosteroids. Molecular docking experiments suggested that (+)-catharanthine binds at the β(+)/α(-) intersubunit interface near the TM2-TM3 loop, where it forms H-bonds with β2-D282 (TM3), β2-K279 (TM2-TM3 loop), and β2-N265 and β2-R269 (TM2). Site-directed mutagenesis experiments supported the in silico results, demonstrating that the K279A and D282A substitutions, that lead to a loss of H-bonding ability of the mutated residue, and the N265S mutation, impair the gating efficacy of (+)-catharanthine. We infer that (+)-catharanthine potentiates the GABAAR through several H-bond interactions with a binding site located in the β(+)/α(-) interface in the transmembrane domain, near the TM2-TM3 loop, where it overlaps with loreclezole binding site.
Keywords: (+)-Catharanthine; Coronaridine congeners; Electrophysiology; Molecular docking; Molecular dynamics; Positive allosteric modulators.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

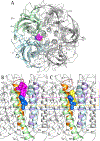



References
-
- Maisonneuve IM, Glick SD, Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment, Pharmacol Biochem Behav 75(3) (2003) 607–18. - PubMed
-
- Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW Jr., Carlson JN, Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum, Brain Res 657(1–2) (1994) 14–22. - PubMed
-
- Glue P, Cape G, Tunnicliff D, Lockhart M, Lam F, Hung N, Hung CT, Harland S, Devane J, Crockett RS, Howes J, Darpo B, Zhou M, Weis H, Friedhoff L, Ascending Single-Dose, Double-Blind, Placebo-Controlled Safety Study of Noribogaine in Opioid-Dependent Patients, Clin Pharmacol Drug Dev 5(6) (2016) 460–468. - PubMed
-
- Brown TK, Alper K, Treatment of opioid use disorder with ibogaine: detoxification and drug use outcomes, Am J Drug Alcohol Abuse 44(1) (2018) 24–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

