A Specific Calprotectin Neo-epitope [CPa9-HNE] in Serum from Inflammatory Bowel Disease Patients Is Associated with Neutrophil Activity and Endoscopic Severity
- PMID: 35304895
- PMCID: PMC9455793
- DOI: 10.1093/ecco-jcc/jjac047
A Specific Calprotectin Neo-epitope [CPa9-HNE] in Serum from Inflammatory Bowel Disease Patients Is Associated with Neutrophil Activity and Endoscopic Severity
Abstract
Background and aims: Endoscopy and the use of faecal calprotectin [faecal CP] are among the least-favoured methods for assessing disease activity by inflammatory bowel disease [IBD] patients; the handling/processing of faecal samples is also impractical. Therefore, we sought to develop a novel neo-epitope serum calprotectin enzyme-linked immunosorbent assay [ELISA], CPa9-HNE, with the aim of quantifying neutrophil activity and neutrophil extracellular trap [NET]-osis and proposing a non-invasive method for monitoring disease activity in IBD patients.
Methods: In vitro cleavage was performed by mixing calprotectin [S100A9/S100A8] with human neutrophil elastase [HNE], and a novel HNE-derived calprotectin neo-epitope [CPa9-HNE] was identified by mass spectrometry for ELISA development. The CPa9-HNE ELISA was quantified in supernatants from ex vivo activated neutrophils and serum samples from patients with ulcerative colitis [UC, n = 43], Crohn's disease [CD, n = 93], and healthy subjects [HS, n = 23]. For comparison, faecal CP and MRP8/14 biomarkers were also measured.
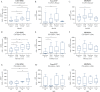
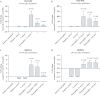
Results: CPa9-HNE was specific for activated neutrophils ex vivo. Serum CPa9-HNE levels were 4-fold higher in CD [p <0.0001] and UC [p <0.0001] patients than in HS. CPa9-HNE correlated well with the Simple Endoscopic Score [SES]-CD score [r = 0.61, p <0.0001], MES [r = 0.46, p = 0.0141], and the full Mayo score [r = 0.52, p = 0.0013]. CPa9-HNE was able to differentiate between CD and UC patients in endoscopic remission and moderate/severe disease activity (CD: area under the curve [AUC] = 0.82 [p = 0.0003], UC: AUC = 0.87 [p = 0.0004]). The performance of CPa9-HNE was equipotent or slightly better than that of faecal CP.
Conclusions: Serum CPa9-HNE levels were highly associated with CD and UC patients. CPa9-HNE correlated with the SES-CD score and the full Mayo score, indicating a strong association with disease activity.
Keywords: Calprotectin; IBD; biomarkers; inflammation; neutrophil elastase; neutrophil extracellular traps [NETs]; neutrophil granulocyte.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Figures



References
-
- D’Haens G, Kelly O, Battat R, et al. Development and validation of a test to monitor endoscopic activity in patients with Crohn’s disease based on serum levels of proteins. Gastroenterology 2020;158:515–26.e10. - PubMed
-
- Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. - PubMed
-
- Coskun M, Vermeire S, Nielsen OH.. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

