Mitochondrial uncoupling attenuates sarcopenic obesity by enhancing skeletal muscle mitophagy and quality control
- PMID: 35304976
- PMCID: PMC9178352
- DOI: 10.1002/jcsm.12982
Mitochondrial uncoupling attenuates sarcopenic obesity by enhancing skeletal muscle mitophagy and quality control
Abstract
Background: Sarcopenic obesity is a highly prevalent disease with poor survival and ineffective medical interventions. Mitochondrial dysfunction is purported to be central in the pathogenesis of sarcopenic obesity by impairing both organelle biogenesis and quality control. We have previously identified that a mitochondrial-targeted furazano[3,4-b]pyrazine named BAM15 is orally available and selectively lowers respiratory coupling efficiency and protects against diet-induced obesity in mice. Here, we tested the hypothesis that mitochondrial uncoupling simultaneously attenuates loss of muscle function and weight gain in a mouse model of sarcopenic obesity.
Methods: Eighty-week-old male C57BL/6J mice with obesity were randomized to 10 weeks of high fat diet (CTRL) or BAM15 (BAM15; 0.1% w/w in high fat diet) treatment. Body weight and food intake were measured weekly. Body composition, muscle function, energy expenditure, locomotor activity, and glucose tolerance were determined after treatment. Skeletal muscle was harvested and evaluated for histology, gene expression, protein signalling, and mitochondrial structure and function.
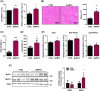
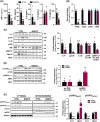
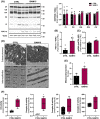
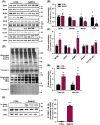
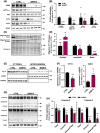
Results: BAM15 decreased body weight (54.0 ± 2.0 vs. 42.3 ± 1.3 g, P < 0.001) which was attributable to increased energy expenditure (10.1 ± 0.1 vs. 11.3 ± 0.4 kcal/day, P < 0.001). BAM15 increased muscle mass (52.7 ± 0.4 vs. 59.4 ± 1.0%, P < 0.001), strength (91.1 ± 1.3 vs. 124.9 ± 1.2 g, P < 0.0001), and locomotor activity (347.0 ± 14.4 vs. 432.7 ± 32.0 m, P < 0.001). Improvements in physical function were mediated in part by reductions in skeletal muscle inflammation (interleukin 6 and gp130, both P < 0.05), enhanced mitochondrial function, and improved endoplasmic reticulum homeostasis. Specifically, BAM15 activated mitochondrial quality control (PINK1-ubiquitin binding and LC3II, P < 0.01), increased mitochondrial activity (citrate synthase and complex II activity, all P < 0.05), restricted endoplasmic reticulum (ER) misfolding (decreased oligomer A11 insoluble/soluble ratio, P < 0.0001) while limiting ER stress (decreased PERK signalling, P < 0.0001), apoptotic signalling (decreased cytochrome C release and Caspase-3/9 activation, all P < 0.001), and muscle protein degradation (decreased 14-kDa actin fragment insoluble/soluble ratio, P < 0.001).
Conclusions: Mitochondrial uncoupling by agents such as BAM15 may mitigate age-related decline in muscle mass and function by molecular and cellular bioenergetic adaptations that confer protection against sarcopenic obesity.
Keywords: Ageing; BAM15; Bioenergetics; Mitochondrial uncoupling; Obesity; Sarcopenia.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors report no conflicts of interest related to this work.
Figures



References
-
- Nations U . World Population Ageing 2020 Highlights: Living Arrangements of Older Persons. 2020. https://www.un.org/development/desa/pd/news/world‐population‐ageing‐2020...
-
- Chomentowski P, Dube JJ, Amati F, Stefanovic‐Racic M, Zhu S, Toledo FG, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction‐induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci 2009;64:575–580. - PMC - PubMed
-
- Bachettini NP, Bielemann RM, Barbosa‐Silva TG, Menezes AMB, Tomasi E, Gonzalez MC. Sarcopenia as a mortality predictor in community‐dwelling older adults: a comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur J Clin Nutr 2020;74:573–580. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

