A GWAS in Idiopathic/Unexplained Infertile Men Detects a Genomic Region Determining Follicle-Stimulating Hormone Levels
- PMID: 35305013
- PMCID: PMC9282256
- DOI: 10.1210/clinem/dgac165
A GWAS in Idiopathic/Unexplained Infertile Men Detects a Genomic Region Determining Follicle-Stimulating Hormone Levels
Abstract
Context: Approximately 70% of infertile men are diagnosed with idiopathic (abnormal semen parameters) or unexplained (normozoospermia) infertility, with the common feature of lacking etiologic factors. Follicle-stimulating hormone (FSH) is essential for initiation and maintenance of spermatogenesis. Certain single-nucleotide variations (SNVs; formerly single-nucleotide polymorphisms [SNPs]) (ie, FSHB c.-211G > T, FSHR c.2039A > G) are associated with FSH, testicular volume, and spermatogenesis. It is unknown to what extent other variants are associated with FSH levels and therewith resemble causative factors for infertility.
Objective: We aimed to identify further genetic determinants modulating FSH levels in a cohort of men presenting with idiopathic or unexplained infertility.
Methods: We retrospectively (2010-2018) selected 1900 men with idiopathic/unexplained infertility. In the discovery study (n = 760), a genome-wide association study (GWAS) was performed (Infinium PsychArrays) in association with FSH values (Illumina GenomeStudio, v2.0). Minor allele frequencies (MAFs) were analyzed for the discovery and an independent normozoospermic cohort. In the validation study (n = 1140), TaqMan SNV polymerase chain reaction was conducted for rs11031005 and rs10835638 in association with andrological parameters.
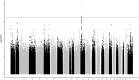
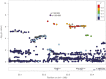
Results: Imputation revealed 9 SNVs in high linkage disequilibrium, with genome-wide significance (P < 4.28e-07) at the FSHB locus 11p.14.1 being associated with FSH. The 9 SNVs accounted for up to a 4.65% variance in FSH level. In the oligozoospermic subgroup, this was increased up to 6.95% and the MAF was enhanced compared to an independent cohort of normozoospermic men. By validation, a significant association for rs11031005/rs10835638 with FSH (P = 4.71e-06/5.55e-07) and FSH/luteinizing hormone ratio (P = 2.08e-12/6.4e-12) was evident.
Conclusions: This GWAS delineates the polymorphic FSHB genomic region as the main determinant of FSH levels in men with unexplained or idiopathic infertility. Given the essential role of FSH, molecular detection of one of the identified SNVs that causes lowered FSH and therewith decreases spermatogenesis could resolve the idiopathic/unexplained origin by this etiologic factor.
Keywords: follicle-stimulating hormone (FSH); genome-wide association study (GWAS); idiopathic male infertility; single-nucleotide variation (SNV).
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

Comment in
-
Male Infertility.J Urol. 2023 Mar;209(3):618-620. doi: 10.1097/JU.0000000000003100. Epub 2022 Dec 13. J Urol. 2023. PMID: 36511615 No abstract available.
References
-
- Kliesch S. Diagnosis of male infertility: diagnostic work-up of the infertile man. Eur Urol Suppl. 2014;13:73-82.
-
- Schlegel PN, Sigman M, Collura B, et al. . Diagnosis and treatment of infertility in men: AUA/ASRM guideline 2021. J Urol. 2021;205(1):36-43. - PubMed
-
- Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol. 2012;38(5):576-594. - PubMed
-
- Sigman M, Lipshultz LI, Howards SS. Office evaluation of the subfertile male. In: Lipshultz LI, Howards SS, Niederberger CS, eds. Infertility in the Male. Cambridge University Press; 2009:176.

