Evaluation of Targeted Next-Generation Sequencing for the Management of Patients Diagnosed with a Cancer of Unknown Primary
- PMID: 35305098
- PMCID: PMC8842368
- DOI: 10.1093/oncolo/oyab014
Evaluation of Targeted Next-Generation Sequencing for the Management of Patients Diagnosed with a Cancer of Unknown Primary
Abstract
Background: Cancer of unknown primary (CUP) comprises a heterogeneous collection of malignancies that are typically associated with a poor prognosis and a lack of effective treatment options. We retrospectively evaluated the clinical utility of targeted next-generation sequencing (NGS) among CUP patients to assist with diagnosis and identify opportunities for molecularly guided therapy.
Patients and methods: Patients with a CUP at Moffitt Cancer Center who underwent NGS between January 1, 2014 and December 31, 2019, were eligible for study inclusion. Next-generation sequencing results were assessed to determine the frequency of clinically actionable molecular alterations, and chart reviews were performed to ascertain the number of patients receiving molecularly guided therapy.
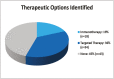
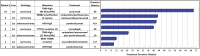
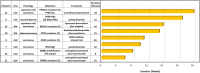
Results: Ninety-five CUP patients were identified for analysis. Next-generation sequencing testing identified options for molecularly guided therapy for 55% (n = 52) of patients. Among patients with molecularly guided therapy options, 33% (n = 17) were prescribed a molecularly guided therapy. The median overall survival for those receiving molecularly guided therapy was 23.6 months. Among the evaluable patients, the median duration of treatment for CUP patients (n = 7) receiving molecular-guided therapy as a first-line therapy was 39 weeks. The median duration of treatment for CUP patients (n = 8) treated with molecularly guided therapy in the second- or later-line setting was 13 weeks. Next-generation sequencing results were found to be suggestive of a likely primary tumor type for 15% (n = 14) of patients.
Conclusion: Next-generation sequencing results enabled the identification of treatment options in a majority of patients and assisted with the identification of a likely primary tumor type in a clinically meaningful subset of patients.
Keywords: cancer genetics; cancer of unknown primary; next generation sequencing; pharmacogenetics; precision medicine.
© The Author(s) 2022. Published by Oxford University Press.
Figures


References
-
- Pavlidis N, Pentheroudakis G.. Cancer of unknown primary site. Lancet. 2012;379(9824):1428-1435. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. - PubMed
-
- National Comprehensive Cancer Network. N.C.C.N. Guidelines. Occult primary. Version 2.2021.
-
- Golfinopoulos V, Pentheroudakis G, Salanti G, Nearchou AD, Ioannidis JP, Pavlidis N.. Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev. 2009;35(7):570-573. - PubMed
-
- Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G; ESMO Guidelines Committee . Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v133-v138. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

