Hexokinase 1 cellular localization regulates the metabolic fate of glucose
- PMID: 35305311
- PMCID: PMC8995391
- DOI: 10.1016/j.molcel.2022.02.028
Hexokinase 1 cellular localization regulates the metabolic fate of glucose
Abstract
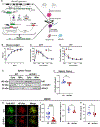
The product of hexokinase (HK) enzymes, glucose-6-phosphate, can be metabolized through glycolysis or directed to alternative metabolic routes, such as the pentose phosphate pathway (PPP) to generate anabolic intermediates. HK1 contains an N-terminal mitochondrial binding domain (MBD), but its physiologic significance remains unclear. To elucidate the effect of HK1 mitochondrial dissociation on cellular metabolism, we generated mice lacking the HK1 MBD (ΔE1HK1). These mice produced a hyper-inflammatory response when challenged with lipopolysaccharide. Additionally, there was decreased glucose flux below the level of GAPDH and increased upstream flux through the PPP. The glycolytic block below GAPDH is mediated by the binding of cytosolic HK1 with S100A8/A9, resulting in GAPDH nitrosylation through iNOS. Additionally, human and mouse macrophages from conditions of low-grade inflammation, such as aging and diabetes, displayed increased cytosolic HK1 and reduced GAPDH activity. Our data indicate that HK1 mitochondrial binding alters glucose metabolism through regulation of GAPDH.
Keywords: GAPDH; S-nitrosylation; hexokinase; inflammation; innate immunity; macrophage; metabolism; mitochondria; pentose phosphate pathway; subcellular localization.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






Comment in
-
Location matters: hexokinase 1 in glucose metabolism and inflammation.Trends Endocrinol Metab. 2022 Oct;33(10):665-667. doi: 10.1016/j.tem.2022.07.005. Epub 2022 Aug 8. Trends Endocrinol Metab. 2022. PMID: 35953432
References
-
- Aflalo C, and Azoulay H (1998). Binding of Rat Brain Hexokinase to Recombinant Yeast Mitochondria: Effect of Environmental Factors and the Source of Porin. J. Bioenerg. Biomembr. 30, 245–255. - PubMed
-
- Alonso-Castro AJ, and Salazar-Olivo LA (2008). The anti-diabetic properties of Guazuma ulmifolia Lam are mediated by the stimulation of glucose uptake in normal and diabetic adipocytes without inducing adipogenesis. J. Ethnopharmacol. 118, 252–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

