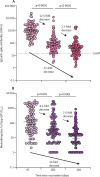
Antibody durability at 1 year after Sputnik V vaccination
- PMID: 35305316
- PMCID: PMC8926411
- DOI: 10.1016/S1473-3099(22)00176-1
Antibody durability at 1 year after Sputnik V vaccination
Conflict of interest statement
LS, SOR, MP, and DSO contributed equally. We declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

