Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer's disease: a longitudinal observational study
- PMID: 35305339
- PMCID: PMC8926925
- DOI: 10.1016/S1474-4422(22)00027-8
Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer's disease: a longitudinal observational study
Erratum in
-
Correction to Lancet Neurol 2022; 21: 329-41.Lancet Neurol. 2022 May;21(5):e5. doi: 10.1016/S1474-4422(22)00140-5. Lancet Neurol. 2022. PMID: 35429484 Free PMC article. No abstract available.
Abstract
Background: Therapeutic modulation of TREM2-dependent microglial function might provide an additional strategy to slow the progression of Alzheimer's disease. Although studies in animal models suggest that TREM2 is protective against Alzheimer's pathology, its effect on tau pathology and its potential beneficial role in people with Alzheimer's disease is still unclear. Our aim was to study associations between the dynamics of soluble TREM2, as a biomarker of TREM2 signalling, and amyloid β (Aβ) deposition, tau-related pathology, neuroimaging markers, and cognitive decline, during the progression of autosomal dominant Alzheimer's disease.
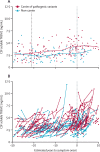
Methods: We did a longitudinal analysis of data from the Dominantly Inherited Alzheimer Network (DIAN) observational study, which includes families with a history of autosomal dominant Alzheimer's disease. Participants aged over 18 years who were enrolled in DIAN between Jan 1, 2009, and July 31, 2019, were categorised as either carriers of pathogenic variants in PSEN1, PSEN2, and APP genes (n=155) or non-carriers (n=93). We measured amounts of cleaved soluble TREM2 using a novel immunoassay in CSF samples obtained every 2 years from participants who were asymptomatic (Clinical Dementia Rating [CDR]=0) and annually for those who were symptomatic (CDR>0). CSF concentrations of Aβ40, Aβ42, total tau (t-tau), and tau phosphorylated on threonine 181 (p-tau) were measured by validated immunoassays. Predefined neuroimaging measurements were total cortical uptake of Pittsburgh compound B PET (PiB-PET), cortical thickness in the precuneus ascertained by MRI, and hippocampal volume determined by MRI. Cognition was measured using a validated cognitive composite (including DIAN word list test, logical memory delayed recall, digit symbol coding test [total score], and minimental status examination). We based our statistical analysis on univariate and bivariate linear mixed effects models.
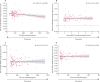
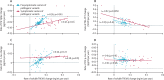
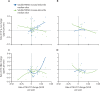
Findings: In carriers of pathogenic variants, a high amyloid burden at baseline, represented by low CSF Aβ42 (β=-4·28 × 10-2 [SE 0·013], p=0·0012), but not high cortical uptake in PiB-PET (β=-5·51 × 10-3 [0·011], p=0·63), was the only predictor of an augmented annual rate of subsequent increase in soluble TREM2. Augmented annual rates of increase in soluble TREM2 were associated with a diminished rate of decrease in amyloid deposition, as measured by Aβ42 in CSF (r=0·56 [0·22], p=0·011), in presymptomatic carriers of pathogenic variants, and with diminished annual rate of increase in PiB-PET (r=-0·67 [0·25], p=0·0060) in symptomatic carriers of pathogenic variants. Presymptomatic carriers of pathogenic variants with annual rates of increase in soluble TREM2 lower than the median showed a correlation between enhanced annual rates of increase in p-tau in CSF and augmented annual rates of increase in PiB-PET signal (r=0·45 [0·21], p=0·035), that was not observed in those with rates of increase in soluble TREM2 higher than the median. Furthermore, presymptomatic carriers of pathogenic variants with rates of increase in soluble TREM2 above or below the median had opposite associations between Aβ42 in CSF and PiB-PET uptake when assessed longitudinally. Augmented annual rates of increase in soluble TREM2 in presymptomatic carriers of pathogenic variants correlated with decreased cortical shrinkage in the precuneus (r=0·46 [0·22]), p=0·040) and diminished cognitive decline (r=0·67 [0·22], p=0·0020).
Interpretation: Our findings in autosomal dominant Alzheimer's disease position the TREM2 response within the amyloid cascade immediately after the first pathological changes in Aβ aggregation and further support the role of TREM2 on Aβ plaque deposition and compaction. Furthermore, these findings underpin a beneficial effect of TREM2 on Aβ deposition, Aβ-dependent tau pathology, cortical shrinkage, and cognitive decline. Soluble TREM2 could, therefore, be a key marker for clinical trial design and interpretation. Efforts to develop TREM2-boosting therapies are ongoing.
Funding: German Research Foundation, US National Institutes of Health.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests HZ has served at scientific advisory boards for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, and CogRx, and has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen. MS-C has served as a consultant and at advisory boards for Roche Diagnostics International and has given lectures in symposia sponsored by Roche Diagnostics, Sociedad Limitada Unipersonal, and Roche Farma, Sociedad Anónima. AMF participates in the scientific advisory boards for Roche Diagnostics, Genentech, and DiamiR, and collaborates as a consultant for DiamiR and Siemens Healthcare Diagnostics. TLSB collaborates with Biogen and Siemens as a consultant and participates in the Advisory board of Eisai and Biogen. JH collaborates as a consultant for Roche and Parabon Nanolabs, and participates in the Advisory board of Eisai, CaringBridge, and WallE. EM collaborates as a consultant for Eli Lilly, has received funding for attending meetings from the Alzheimer Association and Foundation Alzheimer, and participates in the Data Safety Monitoring Board of Eli Lilly, Alector, and in the Advisory Board of Fondation Alzheimer, and Alzmend. KS received royalties for co-developing the therapeutic anti-TREM2 mouse antibody 4D9. JCM has served as consultant for Barcelona Beta Brain Research Center, TS Srinivasan Advisory Board (Chennai, India), has received honoraria for lectures given at Montefiori Grand Rounds (NY, USA) and Tetra-Institute Alzheimer Disease Research Center Seminar Series, and participates in the Advisory Boards of Cure Alzheimer's Fund Research Strategy Council and Leads Advisory Board (IN, USA). KB collaborates as a consultant for Abcam, Axon, BioArctic, Biogen, Japanese Organization for Medical Device Development and Shimadzu, Lilly, MagQu, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, has received honoraria for lectures from Grupo de Estudos de Envelhecimento Cerebral e Demência and Roche Diagnostics, and IFCC and SNIBE, has served at data monitoring committees for Julius Clinical and Novartis, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of Gothenburg University Ventures Incubator programme. JL participates as a consultant in Axon Neuroscience and Biogen, has received honoraria for lectures given by Bayer Vital and Roche, support for attending meetings by AbbVie and Biogen, and has participated in the Advisory board of Axon Neuroscience. JL has also received author fees from Thieme medical publishers, W Kohlhammer GmbH medical publishers, and a compensation for duty as part-time chief marketing officer by MODAG GmbH. RJB collaborates as a consultant with Eisai, Amgen, and Hoffman La-Roche, has received travel support from Hoffman La-Roche, and participates in the C2N Diagnostics Scientific Advisory board. RJB also serves as principal investigator of the Dominantly Inherited Alzheimer's Network-Treatment Unit (DIAN-TU), which is supported by the Alzheimer's Association, GHR Foundation, an anonymous organisation, and the DIAN-TU Pharma Consortium (active members include Eli Lilly and Company, Avid Radiopharmaceuticals, F Hoffman-La Roche, Genentech, Biogen, Eisai, and Janssen. Previous members include Abbvie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, and United Neuroscience). In addition, in-kind support has been received from CogState and Signant Health. CH collaborates with Denali Therapeutics, and has participated on one advisory board meeting of Biogen. CH is also chief advisor of ISAR Bioscience and a member of the scientific advisory board of AviadoBio. CH has received honoraria for lectures at Weill Cornell Medicine, Sheikh Hamdan Webinar Series, Washington University, Eisai, and UT Southwestern Medical Center, and participates in the US Patent Application (number 16/319,373).
Figures
Comment in
-
Dynamics of neuroinflammation in Alzheimer's disease.Lancet Neurol. 2022 Apr;21(4):297-298. doi: 10.1016/S1474-4422(22)00087-4. Lancet Neurol. 2022. PMID: 35305329 No abstract available.
-
TREM2 response occurs early in amyloid cascade.Nat Rev Neurol. 2022 May;18(5):251. doi: 10.1038/s41582-022-00658-1. Nat Rev Neurol. 2022. PMID: 35396358 No abstract available.
References
-
- Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276–1290.e17. - PubMed
-
- Kleinberger G, Yamanishi Y, Suárez-Calvet M, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

