Regorafenib-induced renal-limited thrombotic microangiopathy: a case report and review of literatures
- PMID: 35305559
- PMCID: PMC8933930
- DOI: 10.1186/s12882-021-02656-9
Regorafenib-induced renal-limited thrombotic microangiopathy: a case report and review of literatures
Abstract
Background: Regorafenib belongs to a sub-group of small-molecule multi-targeted tyrosine kinase inhibitors(TKIs). In various studies with respect to the side-effect of regorafenib, drug-associated proteinuria standardly qualified to be defined as nephrotic syndrome was rarely reported as well as the relation of regorafenib with the occurrence and development of thrombotic microangiopathy (TMA).
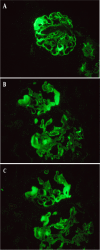
Case presentation: In this case report and literature review, we presented a 62-year-old patient receiving regorafenib for metastatic colon cancer, manifesting abundant proteinuria, in which TMA was also diagnosed through renal biopsy. As far as we were concerned, this was the first reported in terms of regorafenib-induced TMA confirmed by renal biopsy.
Conclusion: This case indicates that regorafenib, a kind of TKIs may result in TMA, which is a rare but life-threatening complication of cancer treatment drug. Insights from this case might help physicians diagnose rare forms of TMA and adjust treatment for patients in a timely manner.
Keywords: Case report; Nephrotic syndrome; Regorafenib; Thrombotic microangiopathy (TMA); Tyrosine kinase inhibitors (TKI).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity[J] Int J Cancer. 2011;129(1):245–255. doi: 10.1002/ijc.25864. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

