Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study
- PMID: 35305740
- PMCID: PMC8930006
- DOI: 10.1016/S0140-6736(22)00007-1
Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study
Abstract
Background: We aimed to assess the effectiveness of a single dose of the Ad26.COV2.S vaccine (Johnson & Johnson) in health-care workers in South Africa during two waves of the South African COVID-19 epidemic.
Methods: In the single-arm, open-label, phase 3B implementation Sisonke study, health-care workers aged 18 years and older were invited for vaccination at one of 122 vaccination sites nationally. Participants received a single dose of 5 × 1010 viral particles of the Ad26.COV2.S vaccine. Vaccinated participants were linked with their person-level data from one of two national medical insurance schemes (scheme A and scheme B) and matched for COVID-19 risk with an unvaccinated member of the general population. The primary outcome was vaccine effectiveness against severe COVID-19, defined as COVID-19-related admission to hospital, hospitalisation requiring critical or intensive care, or death, in health-care workers compared with the general population, ascertained 28 days or more after vaccination or matching, up to data cutoff. This study is registered with the South African National Clinical Trial Registry, DOH-27-022021-6844, ClinicalTrials.gov, NCT04838795, and the Pan African Clinical Trials Registry, PACTR202102855526180, and is closed to accrual.
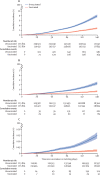
Findings: Between Feb 17 and May 17, 2021, 477 102 health-care workers were enrolled and vaccinated, of whom 357 401 (74·9%) were female and 119 701 (25·1%) were male, with a median age of 42·0 years (33·0-51·0). 215 813 vaccinated individuals were matched with 215 813 unvaccinated individuals. As of data cutoff (July 17, 2021), vaccine effectiveness derived from the total matched cohort was 83% (95% CI 75-89) to prevent COVID-19-related deaths, 75% (69-82) to prevent COVID-19-related hospital admissions requiring critical or intensive care, and 67% (62-71) to prevent COVID-19-related hospitalisations. The vaccine effectiveness for all three outcomes were consistent across scheme A and scheme B. The vaccine effectiveness was maintained in older health-care workers and those with comorbidities including HIV infection. During the course of the study, the beta (B.1.351) and then the delta (B.1.617.2) SARS-CoV-2 variants of concerns were dominant, and vaccine effectiveness remained consistent (for scheme A plus B vaccine effectiveness against COVID-19-related hospital admission during beta wave was 62% [95% CI 42-76] and during delta wave was 67% [62-71], and vaccine effectiveness against COVID-19-related death during beta wave was 86% [57-100] and during delta wave was 82% [74-89]).
Interpretation: The single-dose Ad26.COV2.S vaccine shows effectiveness against severe COVID-19 disease and COVID-19-related death after vaccination, and against both beta and delta variants, providing real-world evidence for its use globally.
Funding: National Treasury of South Africa, the National Department of Health, Solidarity Response Fund NPC, The Michael & Susan Dell Foundation, The Elma Vaccines and Immunization Foundation, and the Bill & Melinda Gates Foundation.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests L-GB declares honoraria for advisory roles from MSD, ViiV Health Care, and Gilead. RJL declares Department of Science and Innovation and South African Medical Research Council (SAMRC) funding to the KwaZulu-Natal Research Innovation and Sequencing Platform at the University of KwaZulu-Natal for the Network for Genomic Surveillance South Africa, which supported the genomic sequencing for this study; and committee membership of the Ministerial Advisory Committee on COVID-19 Vaccines (a committee that makes recommendations to the Minister of South Africa on the national COVID-19 vaccine programme. CC declares grants or contracts from CDC, PATH, the Bill & Melinda Gates Foundation, SAMRC, Wellcome Trust, and Sanofi Pasteur in the past 36 months. MG reports grants from SAMRC during the conduct of the study, and grants from the Bill & Melinda Gates Foundation outside of the submitted work. DBa reports grants from US National Institutes of Health (NIH) and Janssen during the conduct of the study; grants from Defense Advanced Research projects Agency, Massachusetts Consortium on Pathogen Readiness, Ragon Institute, the Bill & Melinda Gates Foundation, SAMRC, Henry Jackson Foundation, Musk Foundation, Gilead, Legend Bio, CureVac, Sanofi, Intima Bio, Alkermes, and Zentalis; and personal fees from SZQ Bio, Pfizer, Celsion, Avidea, Laronde, Meissa, and Vector Sciences outside of the submitted work DBr has three patents (63/121,482; 63/133,969; 63/135,182) licensed to Janssen. All other authors declare no competing interests.
Figures
Comment in
-
Sisonke: reaching several goals together.Lancet. 2022 Mar 19;399(10330):1095-1097. doi: 10.1016/S0140-6736(22)00482-2. Lancet. 2022. PMID: 35305727 Free PMC article. No abstract available.
References
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

