Immune response and safety of heterologous ChAdOx1-nCoV-19/mRNA-1273 vaccination compared with homologous ChAdOx1-nCoV-19 or homologous mRNA-1273 vaccination
- PMID: 35305895
- PMCID: PMC8926322
- DOI: 10.1016/j.jfma.2022.02.020
Immune response and safety of heterologous ChAdOx1-nCoV-19/mRNA-1273 vaccination compared with homologous ChAdOx1-nCoV-19 or homologous mRNA-1273 vaccination
Abstract
Background/purpose: Efficacy and safety data of heterologous prime-boost vaccination against SARS-CoV-2 remains limited.
Methods: We recruited adult volunteers for homologous or heterologous prime-boost vaccinations with adenoviral (ChAdOx1, AstraZeneca) and/or mRNA (mRNA-1273, Moderna) vaccines. Four groups of prime-boost vaccination schedules were designed: Group 1, ChAdOx1/ChAdOx1 8 weeks apart; Group 2, ChAdOx1/mRNA-1273 8 weeks apart; Group 3, ChAdOx1/mRNA-1273 4 weeks apart; and Group 4, mRNA-1273/mRNA-1273 4 weeks apart. The primary outcome was serum anti-SARS-CoV-2 IgG titers and neutralizing antibody titers against B.1.1.7 (alpha) and B.1.617.2 (delta) variants on day 28 after the second dose. Adverse events were recorded up until 84 days after the second dose.
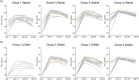
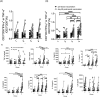
Results: We enrolled 399 participants with a median age of 41 years and 75% were female. On day 28 after the second dose, the anti-SARS-CoV-2 IgG titers of both heterologous vaccinations (Group 2 and Group 3) were significantly higher than that of homologous ChAdOx1 vaccination (Group 1), and comparable with homologous mRNA-1273 vaccination (Group 4). The heterologous vaccination group had better neutralizing antibody responses against the alpha and delta variant as compared to the homologous ChAdOx1 group. Most of the adverse events (AEs) were mild and transient. AEs were less frequent when heterologous boosting was done at 8 weeks rather than at 4 weeks.
Conclusion: Heterologous ChAdOx1/mRNA-1273 vaccination provided higher immunogenicity than homologous ChAdOx1 vaccination and comparable immunogenicity with the homologous mRNA-1273 vaccination. Our results support the safety and efficacy of heterologous prime-boost vaccination using the ChAdOx1 and mRNA-1273 COVID-19 vaccines. (ClinicalTrials.gov number, NCT05074368).
Keywords: Adenovirus-vector vaccine; Coronavirus disease 2019 (COVID-19); Immune response; Messenger RNA vaccine; Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2).
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest relevant to this article.
Figures

References
-
- World Health Organization (WHO) WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
-
- Taiwan Centers for Diseases Control (T-CDC) COVID-19 (SARS-CoV-2 infection) https://www.cdc.gov.tw/En
-
- World Health Organization AZD1222 vaccine against COVID-19 developed by Oxford University and AstraZeneca: background paper. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_reco...
-
- Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet. 2021;397:881–891. - PMC - PubMed
-
- World Health Organization mRNA-1273 vaccine (Moderna) against COVID-19 background document: draft prepared by the strategic advisory group of experts (SAGE) on immunization working group on COVID-19 vaccines. 19 January 2021. https://apps.who.int/iris/handle/10665/338738
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

