Spatial regulation of endosomes in growing dendrites
- PMID: 35306006
- PMCID: PMC10646839
- DOI: 10.1016/j.ydbio.2022.03.004
Spatial regulation of endosomes in growing dendrites
Abstract
Many membrane proteins are highly enriched in either dendrites or axons. This non-uniform distribution is a critical feature of neuronal polarity and underlies neuronal function. The molecular mechanisms responsible for polarized distribution of membrane proteins has been studied for some time and many answers have emerged. A less well studied feature of neurons is that organelles are also frequently non-uniformly distributed. For instance, EEA1-positive early endosomes are somatodendritic whereas synaptic vesicles are axonal. In addition, some organelles are present in both axons and dendrites, but not distributed uniformly along the processes. One well known example are lysosomes which are abundant in the soma and proximal dendrite, but sparse in the distal dendrite and the distal axon. The mechanisms that determine the spatial distribution of organelles along dendrites are only starting to be studied. In this review, we will discuss the cell biological mechanisms of how the distribution of diverse sets of endosomes along the proximal-distal axis of dendrites might be regulated. In particular, we will focus on the regulation of bulk homeostatic mechanisms as opposed to local regulation. We posit that immature dendrites regulate organelle motility differently from mature dendrites in order to spatially organize dendrite growth, branching and sculpting.
Keywords: Dendrite; Dendritogenesis; Directional Transport; Endosome; Lysosome; Organelle Positioning.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures

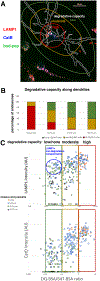


References
-
- Alberi S, Boda B, Steiner P, Nikonenko I, Hirling H, Muller D, 2005. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Mol. Cell. Neurosci. 29 (2), 313–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

