A method for the selective depletion of microglia in the dorsal hippocampus in the juvenile rat brain
- PMID: 35306037
- PMCID: PMC9070732
- DOI: 10.1016/j.jneumeth.2022.109567
A method for the selective depletion of microglia in the dorsal hippocampus in the juvenile rat brain
Abstract
Background: To understand the role of microglia in brain function and development, methods have emerged to deplete microglia throughout the brain. Liposome-encapsulated clodronate (LEC) can be infused into the brain to deplete microglia in a brain-region and time-specific manner.
New method: This study validates methodology to deplete microglia in the rat dorsal hippocampus (dHP) during a specific period of juvenile development. Stereotaxic surgery was performed to infuse LEC at postnatal day (P) 16 or 19 into dHP. Rat brains were harvested at various ages to determine specificity of infusion and duration of depletion.
Results: P19 infusion of LEC into dHP with a 27G syringe depleted microglia in dHP subregions CA1, dentate gyrus (DG), and CA3 from P24-P30. There was also evidence of depletion in parietal cortex above the infusion site. P16 infusion of LEC with a 32 G syringe depleted microglia only in dHP subregions CA1 and DG from P21-P40.
Comparison with existing method(s): Previous methods have infused LEC intra-hippocampally in adult rats or intra-cerebroventricularly in neonatal rats. This study is the first to publish methodology to deplete microglia in a brain-region specific manner during juvenile rat development.
Conclusions: The timing of LEC infusion during the juvenile period can be adjusted to achieve maximal microglia depletion by a specific postnatal day. A 27G needle results in LEC backflow during the infusion, but also allows LEC to reach all subregions of dHP. Infusion with a 32 G needle prevents backflow during infusion, but results in a more local spread of LEC within dHP.
Keywords: Dorsal hippocampus; Hippocampal infusion; Liposome-encapsulated clodronate; Microglia depletion; Stereotaxic surgery.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures
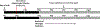



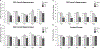
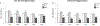


Similar articles
-
Liposome-encapsulated clodronate specifically depletes spinal microglia and reduces initial neuropathic pain.Biochem Biophys Res Commun. 2018 May 15;499(3):499-505. doi: 10.1016/j.bbrc.2018.03.177. Epub 2018 Mar 28. Biochem Biophys Res Commun. 2018. PMID: 29596830
-
Microglial depletion using intrahippocampal injection of liposome-encapsulated clodronate in prolonged hypothermic cardiac arrest in rats.Resuscitation. 2012 Apr;83(4):517-26. doi: 10.1016/j.resuscitation.2011.09.016. Epub 2011 Oct 2. Resuscitation. 2012. PMID: 21970817 Free PMC article.
-
Microglia Regulate Cell Genesis in a Sex-dependent Manner in the Neonatal Hippocampus.Neuroscience. 2021 Jan 15;453:237-255. doi: 10.1016/j.neuroscience.2020.10.009. Epub 2020 Oct 28. Neuroscience. 2021. PMID: 33129890
-
Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats.Behav Brain Res. 2017 Jan 1;316:279-293. doi: 10.1016/j.bbr.2016.09.006. Epub 2016 Sep 6. Behav Brain Res. 2017. PMID: 27613230 Free PMC article.
-
Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics.Neurobiol Learn Mem. 2016 Mar;129:38-49. doi: 10.1016/j.nlm.2015.10.008. Epub 2015 Oct 26. Neurobiol Learn Mem. 2016. PMID: 26514299 Free PMC article. Review.
Cited by
-
Synapse Regulation.Adv Neurobiol. 2024;37:179-208. doi: 10.1007/978-3-031-55529-9_11. Adv Neurobiol. 2024. PMID: 39207693 Review.
References
-
- Biewenga J, van der Ende MB, Krist LFG, Borst A, Ghufron M, & van Rooijen N (1995). Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: Depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of freund’s adjuvant. Cell and Tissue Research, 280(1), 189–196. 10.1007/BF00304524 - DOI - PubMed
-
- Dehghani F, Conrad A, Kohl A, Korf HW, & Hailer NP (2004). Clodronate inhibits the secretion of proinflammatory cytokines and NO by isolated microglial cells and reduces the number of proliferating glial cells in excitotoxically injured organotypic hippocampal slice cultures. Experimental Neurology, 189(2), 241–251. 10.1016/j.expneurol.2004.06.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

